Assessment of tantalum nanoparticle-induced MC3T3-E1 proliferation and underlying mechanisms
- PMID: 34689241
- PMCID: PMC8542006
- DOI: 10.1007/s10856-021-06606-7
Assessment of tantalum nanoparticle-induced MC3T3-E1 proliferation and underlying mechanisms
Abstract
Objective: In our previous study, tantalum nanoparticle (Ta-NPs) was demonstrated to promote osteoblast proliferation via autophagy induction, but the specific mechanism remains unclear. In the present study, we will explore the potential mechanism.
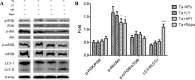
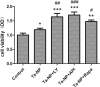
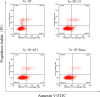
Methods: Ta-NPs was characterized by transmission electron microscopy, scanning electron microscopy, dynamic light scattering, and BET specific surface area test. MC3T3-E1 were treated with 0 or 20 μg/mL Ta-NPs with or without pretreatment with 10 μM LY294002, Triciribine, Rapamycin (PI3K/Akt/mTOR pathway inhibitors) for 1 h respectively. Western blotting was used to detect the expressions of pathway proteins and LC3B. CCK-8 assay was used to assess cell viability. Flow cytometry was used to detect apoptosis and cell cycle.
Results: After pretreatment with LY294002, Triciribine and Rapamycin, the p-Akt/Akt ratio of pathway protein in Triciribine and Rapamycin groups decreased (P < 0.05), while the autophagy protein LC3-II/LC3-I in the Rapamycin group was upregulated obviously (P < 0.001). In all pretreated groups, apoptosis was increased (LY294002 group was the most obvious), G1 phase cell cycle was arrested (Triciribine and Rapamycin groups were more obvious), and MC3T3-E1 cells were proliferated much more (P < 0.01, P < 0.001, P < 0.05).
Conclusion: Pretreatment with Triciribine or Rapamycin has a greater effect on pathway protein Akt, cell cycle arrest, autophagy protein, and cell proliferation but with inconsistent magnitude, which may be inferred that the Akt/mTOR pathway, as well as its feedback loop, were more likely involved in these processes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Involvement of autophagy in tantalum nanoparticle-induced osteoblast proliferation.Int J Nanomedicine. 2017 Jun 7;12:4323-4333. doi: 10.2147/IJN.S136281. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28652735 Free PMC article.
-
High glucose induces autophagy of MC3T3-E1 cells via ROS-AKT-mTOR axis.Mol Cell Endocrinol. 2016 Jul 5;429:62-72. doi: 10.1016/j.mce.2016.03.036. Epub 2016 Apr 8. Mol Cell Endocrinol. 2016. PMID: 27068641
-
LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells.Int J Gynecol Cancer. 2012 Jan;22(1):15-22. doi: 10.1097/IGC.0b013e3182322834. Int J Gynecol Cancer. 2012. PMID: 22080879
-
The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces.J Biomed Mater Res A. 2013 Mar;101(3):748-54. doi: 10.1002/jbm.a.34377. Epub 2012 Aug 31. J Biomed Mater Res A. 2013. PMID: 22941963
-
[Effect of MTRR gene on apoptosis and autophagy pathways in multiresistant epithelial ovarian cancer].Zhonghua Fu Chan Ke Za Zhi. 2016 Apr 25;51(4):285-92. doi: 10.3760/cma.j.issn.0529-567X.2016.04.008. Zhonghua Fu Chan Ke Za Zhi. 2016. PMID: 27116987 Chinese.
Cited by
-
Advances in surface modification of tantalum and porous tantalum for rapid osseointegration: A thematic review.Front Bioeng Biotechnol. 2022 Sep 13;10:983695. doi: 10.3389/fbioe.2022.983695. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36177183 Free PMC article. Review.
-
Advancements in tantalum based nanoparticles for integrated imaging and photothermal therapy in cancer management.RSC Adv. 2024 Oct 23;14(46):33681-33740. doi: 10.1039/d4ra05732e. eCollection 2024 Oct 23. RSC Adv. 2024. PMID: 39450067 Free PMC article. Review.
References
-
- Wang L, Hu X, Ma X, Ma Z, Zhang Y, Lu Y, et al. Promotion of osteointegration under diabetic conditions by tantalum coating-based surface modification on 3-dimensional printed porous titanium implants. Colloids Surf B Biointerfaces. 2016;148:440–52. doi: 10.1016/j.colsurfb.2016.09.018. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

