Unique N-Terminal Interactions Connect F-BOX STRESS INDUCED (FBS) Proteins to a WD40 Repeat-like Protein Pathway in Arabidopsis
- PMID: 34686037
- PMCID: PMC8537223
- DOI: 10.3390/plants10102228
Unique N-Terminal Interactions Connect F-BOX STRESS INDUCED (FBS) Proteins to a WD40 Repeat-like Protein Pathway in Arabidopsis
Abstract
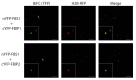
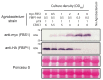
SCF-type E3 ubiquitin ligases provide specificity to numerous selective protein degradation events in plants, including those that enable survival under environmental stress. SCF complexes use F-box (FBX) proteins as interchangeable substrate adaptors to recruit protein targets for ubiquitylation. FBX proteins almost universally have structure with two domains: A conserved N-terminal F-box domain interacts with a SKP protein and connects the FBX protein to the core SCF complex, while a C-terminal domain interacts with the protein target and facilitates recruitment. The F-BOX STRESS INDUCED (FBS) subfamily of plant FBX proteins has an atypical structure, however, with a centrally located F-box domain and additional conserved regions at both the N- and C-termini. FBS proteins have been linked to environmental stress networks, but no ubiquitylation target(s) or biological function has been established for this subfamily. We have identified two WD40 repeat-like proteins in Arabidopsis that are highly conserved in plants and interact with FBS proteins, which we have named FBS INTERACTING PROTEINs (FBIPs). FBIPs interact exclusively with the N-terminus of FBS proteins, and this interaction occurs in the nucleus. FBS1 destabilizes FBIP1, consistent with FBIPs being ubiquitylation targets SCFFBS1 complexes. This work indicates that FBS proteins may function in stress-responsive nuclear events, and it identifies two WD40 repeat-like proteins as new tools with which to probe how an atypical SCF complex, SCFFBS, functions via FBX protein N-terminal interaction events.
Keywords: F-box protein; SCF complex; WD40 repeat-like protein; stress response.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
A comprehensive analysis of interaction and localization of Arabidopsis SKP1-like (ASK) and F-box (FBX) proteins.PLoS One. 2012;7(11):e50009. doi: 10.1371/journal.pone.0050009. Epub 2012 Nov 15. PLoS One. 2012. PMID: 23166809 Free PMC article.
-
The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis.Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11519-24. doi: 10.1073/pnas.162339999. Epub 2002 Aug 8. Proc Natl Acad Sci U S A. 2002. PMID: 12169662 Free PMC article.
-
Identification of SkpA-CulA-F-box E3 ligase complexes in pathogenic Aspergilli.Fungal Genet Biol. 2020 Jul;140:103396. doi: 10.1016/j.fgb.2020.103396. Epub 2020 Apr 20. Fungal Genet Biol. 2020. PMID: 32325169
-
The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction.Prog Biophys Mol Biol. 1999;72(3):299-328. doi: 10.1016/s0079-6107(99)00010-3. Prog Biophys Mol Biol. 1999. PMID: 10581972 Review.
-
Sugar-Recognizing Ubiquitin Ligases: Action Mechanisms and Physiology.Front Physiol. 2019 Feb 19;10:104. doi: 10.3389/fphys.2019.00104. eCollection 2019. Front Physiol. 2019. PMID: 30837888 Free PMC article. Review.
Cited by
-
Characterization key genes of Arabidopsis seedlings in response to β-caryophyllene, eugenol using combined transcriptome and WGCN analysis.Front Plant Sci. 2024 Jan 4;14:1295779. doi: 10.3389/fpls.2023.1295779. eCollection 2023. Front Plant Sci. 2024. PMID: 38239209 Free PMC article.
-
What Makes the Life of Stressed Plants a Little Easier? Defense Mechanisms against Adverse Conditions.Plants (Basel). 2023 Feb 24;12(5):1040. doi: 10.3390/plants12051040. Plants (Basel). 2023. PMID: 36903901 Free PMC article.
-
The Identification and Analysis of the Self-Incompatibility Pollen Determinant Factor SLF in Lycium barbarum.Plants (Basel). 2024 Mar 26;13(7):959. doi: 10.3390/plants13070959. Plants (Basel). 2024. PMID: 38611487 Free PMC article.
References
-
- Wang P., Nolan T.M., Clark N.M., Jiang H., Montes-Serey C., Guo H., Bassham D.C., Walley J.W., Yin Y. The F-Box E3 Ubiquitin Ligase BAF1 Mediates the Degradation of the Brassinosteroid-Activated Transcription Factor BES1 through Selective Autophagy in Arabidopsis. Plant Cell. 2021:koab210. doi: 10.1093/plcell/koab210. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

