Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals?
- PMID: 34685524
- PMCID: PMC8533771
- DOI: 10.3390/cells10102544
Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals?
Abstract
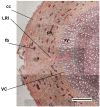
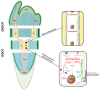
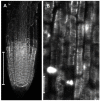
Mechanical stress in tree roots induces the production of reaction wood (RW) and the formation of new branch roots, both functioning to avoid anchorage failure and limb damage. The vascular cambium (VC) is the factor responsible for the onset of these responses as shown by their occurrence when all primary tissues and the root tips are removed. The data presented confirm that the VC is able to evaluate both the direction and magnitude of the mechanical forces experienced before coordinating the most fitting responses along the root axis whenever and wherever these are necessary. The coordination of these responses requires intense crosstalk between meristematic cells of the VC which may be very distant from the place where the mechanical stress is first detected. Signaling could be facilitated through plasmodesmata between meristematic cells. The mechanism of RW production also seems to be well conserved in the stem and this fact suggests that the VC could behave as a single structure spread along the plant body axis as a means to control the relationship between the plant and its environment. The observation that there are numerous morphological and functional similarities between different meristems and that some important regulatory mechanisms of meristem activity, such as homeostasis, are common to several meristems, supports the hypothesis that not only the VC but all apical, primary and secondary meristems present in the plant body behave as a single interconnected structure. We propose to name this structure "meristematic connectome" given the possibility that the sequence of meristems from root apex to shoot apex could represent a pluricellular network that facilitates long-distance signaling in the plant body. The possibility that the "meristematic connectome" could act as a single structure active in adjusting the plant body to its surrounding environment throughout the life of a plant is now proposed.
Keywords: Arabidopsis thaliana L.; Populus nigra L.; connectome; meristems; root apical meristem; root procambial bundles; shoot apical meristem; vascular cambium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The vascular cambium: molecular control of cellular structure.Protoplasma. 2010 Dec;247(3-4):145-61. doi: 10.1007/s00709-010-0211-z. Epub 2010 Oct 27. Protoplasma. 2010. PMID: 20978810 Review.
-
Genome-wide analysis of long non-coding RNAs in shoot apical meristem and vascular cambium in Populus tomentosa.J Plant Physiol. 2022 Aug;275:153759. doi: 10.1016/j.jplph.2022.153759. Epub 2022 Jun 28. J Plant Physiol. 2022. PMID: 35820347
-
The Dynamics of Cambial Stem Cell Activity.Annu Rev Plant Biol. 2019 Apr 29;70:293-319. doi: 10.1146/annurev-arplant-050718-100402. Epub 2019 Mar 1. Annu Rev Plant Biol. 2019. PMID: 30822110 Review.
-
The Populus homeobox gene ARBORKNOX1 reveals overlapping mechanisms regulating the shoot apical meristem and the vascular cambium.Plant Mol Biol. 2006 Aug;61(6):917-32. doi: 10.1007/s11103-006-0059-y. Plant Mol Biol. 2006. PMID: 16927204
-
MOL1 is required for cambium homeostasis in Arabidopsis.Plant J. 2016 May;86(3):210-20. doi: 10.1111/tpj.13169. Epub 2016 Apr 14. Plant J. 2016. PMID: 26991973 Free PMC article.
Cited by
-
Physiological roles of Arabidopsis MCA1 and MCA2 based on their dynamic expression patterns.J Plant Res. 2024 Sep;137(5):785-797. doi: 10.1007/s10265-024-01575-8. Epub 2024 Aug 28. J Plant Res. 2024. PMID: 39196431 Free PMC article. Review.
-
Editorial: Modulation of Growth and Development of Tree Roots in Forest Ecosystems.Front Plant Sci. 2022 Feb 15;13:850163. doi: 10.3389/fpls.2022.850163. eCollection 2022. Front Plant Sci. 2022. PMID: 35242162 Free PMC article. No abstract available.
-
The adaptability of Ulmus pumila and the sensitivity of Populus sibirica to semi-arid steppe is reflected in the stem and root vascular cambium and anatomical wood traits.Front Plant Sci. 2024 Jun 12;15:1393245. doi: 10.3389/fpls.2024.1393245. eCollection 2024. Front Plant Sci. 2024. PMID: 38933456 Free PMC article.
-
Exploring the complex information processes underlying plant behavior.Plant Signal Behav. 2024 Dec 31;19(1):2411913. doi: 10.1080/15592324.2024.2411913. Epub 2024 Oct 9. Plant Signal Behav. 2024. PMID: 39381978 Free PMC article. Review.
-
Key Pathways and Genes of Arabidopsis thaliana and Arabidopsis halleri Roots under Cadmium Stress Responses: Differences and Similarities.Plants (Basel). 2023 Apr 27;12(9):1793. doi: 10.3390/plants12091793. Plants (Basel). 2023. PMID: 37176850 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

