Preparation and Pharmacokinetic Characterization of an Anti-Virulence Compound Nanosuspensions
- PMID: 34683879
- PMCID: PMC8540953
- DOI: 10.3390/pharmaceutics13101586
Preparation and Pharmacokinetic Characterization of an Anti-Virulence Compound Nanosuspensions
Abstract
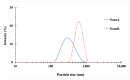
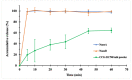
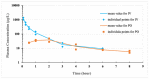
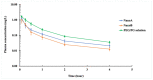
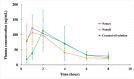
Antibiotic resistance has become a worldwide public health threat due to the rapid evolution and spread of antibiotic-resistant bacteria. CCG-211790 is a novel anti-virulence compound that does not kill bacteria but could ameliorate human diseases by inhibiting expression of virulence factors, thereby applying less selection pressure for antibiotic resistance. However, its potential clinical use is restricted because of its poor aqueous solubility, resulting in formulation challenges. Nanosuspension technology is an effective way to circumvent this problem. Nanosuspensions of CCG-211790 with two different particle sizes, NanoA (315 ± 6 nm) and NanoB (915 ± 24 nm), were prepared using an antisolvent precipitation-ultrasonication method with Tween 80 as the stabilizer. Particle and pharmacokinetics (PK) of CCG-211790 nanosuspensions were characterized. Both NanoA and NanoB demonstrated remarkable increases in dissolution rate compared with the bulk compound. The PK parameters of NanoA were comparable to those of CCG-211790 solution formulation in intravenous or oral administration, suggesting that CCG-211790 nanosuspensions with smaller particle size improved oral bioavailability and drug exposure compared to traditional formulations of drug candidates.
Keywords: anti-virulence; biofilm; nanosuspension; pharmacokinetic; wound infection.
Conflict of interest statement
D.W.A. and H.L. are currently employed by Ivogen Inc., which is a subsidiary of Nanova, Inc. H.S. (Hongmin Sun) is a consultant of Nanova Inc., Ivogen Inc., and owns stocks in Nanova, Inc. F.Q. is currently employed by BeiGene Ltd. X.H. is currently employed by Frontage Laboratories, Inc. The companies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors would like to declare the following patents associated with this research for methods and compositions for treating bacterial infection: H.S. (Hongmin Sun) is a co-inventor on patents US 8501722, US 9504688, Japan 6293736, and European 2844258; H.S. (Hongmin Sun) and DWA are co-inventors on patent US 9814719; H.S. (Hongmin Sun), D.W.A., and F.Q. are co-inventors on patent US 10441588. Ivogen Inc. is developing products related to these patents. This does not alter the authors’ adherence to all the journal’s policies on sharing data and materials.
Figures

Similar articles
-
Preparation and characterization of amorphous ezetimibe nanosuspensions intended for enhancement of oral bioavailability.Int J Pharm Investig. 2014 Jul;4(3):131-7. doi: 10.4103/2230-973X.138344. Int J Pharm Investig. 2014. PMID: 25126526 Free PMC article.
-
In vitro dissolution and bioavailability study of furosemide nanosuspension prepared using design of experiment (DoE).Saudi Pharm J. 2019 Jan;27(1):96-105. doi: 10.1016/j.jsps.2018.09.002. Epub 2018 Sep 3. Saudi Pharm J. 2019. PMID: 30662312 Free PMC article.
-
Nasal delivery of nanosuspension-based mucoadhesive formulation with improved bioavailability of loratadine: Preparation, characterization, and in vivo evaluation.Int J Pharm. 2020 Apr 15;579:119166. doi: 10.1016/j.ijpharm.2020.119166. Epub 2020 Feb 19. Int J Pharm. 2020. PMID: 32084574
-
Emerging role of nanosuspensions in drug delivery systems.Biomater Res. 2020 Jan 15;24:3. doi: 10.1186/s40824-020-0184-8. eCollection 2020. Biomater Res. 2020. PMID: 31969986 Free PMC article. Review.
-
Production of nanosuspensions as a tool to improve drug bioavailability: focus on topical delivery.Curr Pharm Des. 2015;21(42):6089-103. doi: 10.2174/1381612821666151027152350. Curr Pharm Des. 2015. PMID: 26503149 Review.
Cited by
-
Key Challenges, Influencing Factors, and Future Perspectives of Nanosuspensions in Enhancing Brain Drug Delivery.Curr Pharm Des. 2024;30(32):2524-2537. doi: 10.2174/0113816128317347240625105501. Curr Pharm Des. 2024. PMID: 38988170 Review.
-
Nanosuspension-Based Drug Delivery Systems for Topical Applications.Int J Nanomedicine. 2024 Jan 25;19:825-844. doi: 10.2147/IJN.S447429. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38293608 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

