Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol
- PMID: 34682353
- PMCID: PMC8535641
- DOI: 10.3390/ijerph182010607
Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol
Abstract
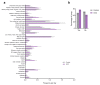
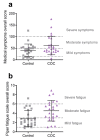
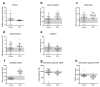
Combined oral contraceptive (COC) use has been associated with various adverse effects. Formulations containing drospirenone (DRSP) and ethinyl estradiol (EE) are generally regarded as milder COCs. Whether long term use of these pills indeed has a low health risk remains questionable. COC use may affect the biotransformation balance by increasing the toxic load or by interfering with the pharmacokinetics of other drugs. This may negatively impact overall health via the production of toxic biotransformation metabolites and induction of oxidative stress. Although individual enzymes involved in biotransformation are known to be regulated by COCs, the effect of COC use on the overall liver biotransformation efficiency has not been reported. Here, we evaluated the general subjective health status and overall liver biotransformation efficiency of healthy young women who were either long term chronic users of COCs containing DRSP/EE, or who were not using any hormonal products. COC users suffered from moderate to severe fatigue and reported more health-related symptoms. Furthermore, phase I (CYP1A2) activity was reduced whereas phase II conjugation reactions (glucuronide conjugation and glycine conjugation) were increased in COC users. Finally, serum peroxide levels were markedly elevated and antioxidant capacity of plasma was reduced in COC users. COCs containing DRSP/EE may, therefore, adversely affect health status and disturb the balance between phase I and II biotransformation reactions. These effects may be mediated by oxidative stress.
Keywords: biotransformation; fatigue; health status; oral contraceptives; oxidative stress.
Conflict of interest statement
G.V., C.L.v.d.B., F.H.v.d.W., and E.E. declare that they have no conflict of interest.
Figures
Similar articles
-
Serum Metabolome Changes in Relation to Prothrombotic State Induced by Combined Oral Contraceptives with Drospirenone and Ethinylestradiol.OMICS. 2020 Jul;24(7):404-414. doi: 10.1089/omi.2020.0009. Epub 2020 May 29. OMICS. 2020. PMID: 32471328
-
The Impact of Two Combined Oral Contraceptives Containing Ethinyl Estradiol and Drospirenone on Whole Blood Clot Viscoelasticity and the Biophysical and Biochemical Characteristics of Erythrocytes.Microsc Microanal. 2018 Dec;24(6):713-728. doi: 10.1017/S1431927618015453. Microsc Microanal. 2018. PMID: 30588913
-
Effects of a new oral contraceptive containing an antimineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism.J Clin Endocrinol Metab. 1995 Jun;80(6):1816-21. doi: 10.1210/jcem.80.6.7775629. J Clin Endocrinol Metab. 1995. PMID: 7775629 Clinical Trial.
-
[Individualization of low-dose oral contraceptives. Pharmacological principles and practical indications for oral contraceptives].Minerva Ginecol. 2007 Aug;59(4):415-25. Minerva Ginecol. 2007. PMID: 17923832 Review. Italian.
-
Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: A literature review.Eur J Contracept Reprod Health Care. 2015;20(5):329-43. doi: 10.3109/13625187.2015.1050091. Epub 2015 May 26. Eur J Contracept Reprod Health Care. 2015. PMID: 26007631 Review.
Cited by
-
The Effect of Menopause on Antipsychotic Response.Brain Sci. 2022 Oct 4;12(10):1342. doi: 10.3390/brainsci12101342. Brain Sci. 2022. PMID: 36291276 Free PMC article. Review.
-
Effect of Iron Depletion by Bloodletting vs. Observation on Oxidative Stress Biomarkers of Women with Functional Hyperandrogenism Taking a Combined Oral Contraceptive: A Randomized Clinical Trial.J Clin Med. 2022 Jul 3;11(13):3864. doi: 10.3390/jcm11133864. J Clin Med. 2022. PMID: 35807149 Free PMC article.
References
-
- United Nations . Contraceptive Use by Method 2019: Data Booklet. Department of Economic and Social Affairs; New York, NY, USA: 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

