Placental Ischemia Says "NO" to Proper NOS-Mediated Control of Vascular Tone and Blood Pressure in Preeclampsia
- PMID: 34681920
- PMCID: PMC8541176
- DOI: 10.3390/ijms222011261
Placental Ischemia Says "NO" to Proper NOS-Mediated Control of Vascular Tone and Blood Pressure in Preeclampsia
Abstract
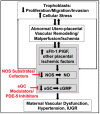
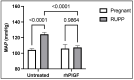
In this review, we first provide a brief overview of the nitric oxide synthase (NOS) isoforms and biochemistry. This is followed by describing what is known about NOS-mediated blood pressure control during normal pregnancy. Circulating nitric oxide (NO) bioavailability has been assessed by measuring its metabolites, nitrite (NO2) and/or nitrate (NO3), and shown to rise throughout normal pregnancy in humans and rats and decline postpartum. In contrast, placental malperfusion/ischemia leads to systemic reductions in NO bioavailability leading to maternal endothelial and vascular dysfunction with subsequent development of hypertension in PE. We end this article by describing emergent risk factors for placental malperfusion and ischemic disease and discussing strategies to target the NOS system therapeutically to increase NO bioavailability in preeclamptic patients. Throughout this discussion, we highlight the critical importance that experimental animal studies have played in our current understanding of NOS biology in normal pregnancy and their use in finding novel ways to preserve this signaling pathway to prevent the development, treat symptoms, or reduce the severity of PE.
Keywords: intrauterine growth restriction; nitric oxide; nitric oxide synthases; potential therapies; preeclampsia; pregnancy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Placental nitric oxide synthase (NOS) activity and nitric oxide (NO) production in normal pregnancy, pre-eclampsia and eclampsia.Int J Gynaecol Obstet. 2001 Feb;72(2):127-33. doi: 10.1016/s0020-7292(00)00314-3. Int J Gynaecol Obstet. 2001. PMID: 11166745
-
The glucagon-like peptide 1 receptor agonist liraglutide attenuates placental ischemia-induced hypertension.Am J Physiol Heart Circ Physiol. 2020 Jan 1;318(1):H72-H77. doi: 10.1152/ajpheart.00486.2019. Epub 2019 Nov 15. Am J Physiol Heart Circ Physiol. 2020. PMID: 31729903 Free PMC article.
-
Estimation of oxidative products of nitric oxide (nitrates, nitrites) in preeclampsia.Aust N Z J Obstet Gynaecol. 1999 Nov;39(4):484-7. doi: 10.1111/j.1479-828x.1999.tb03139.x. Aust N Z J Obstet Gynaecol. 1999. PMID: 10687770
-
Placental nitric oxide metabolism.Reprod Fertil Dev. 1995;7(6):1525-31. doi: 10.1071/rd9951525. Reprod Fertil Dev. 1995. PMID: 8743159 Review.
-
Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease.J Intern Med. 2019 Jan;285(1):2-18. doi: 10.1111/joim.12818. Epub 2018 Aug 28. J Intern Med. 2019. PMID: 30039620 Review.
Cited by
-
Paradoxes: Cholesterol and Hypoxia in Preeclampsia.Biomolecules. 2024 Jun 13;14(6):691. doi: 10.3390/biom14060691. Biomolecules. 2024. PMID: 38927094 Free PMC article. Review.
-
Arginase-1 promotes lens epithelial-to-mesenchymal transition in different models of anterior subcapsular cataract.Cell Commun Signal. 2023 Sep 18;21(1):236. doi: 10.1186/s12964-023-01210-4. Cell Commun Signal. 2023. PMID: 37723490 Free PMC article.
-
Ultrasound based radiomics model for assessment of placental function in pregnancies with preeclampsia.Sci Rep. 2024 Sep 10;14(1):21123. doi: 10.1038/s41598-024-72046-2. Sci Rep. 2024. PMID: 39256496 Free PMC article.
-
Sodium Nitrite Attenuates Reduced Activity of Vascular Matrix Metalloproteinase-2 and Vascular Hyper-Reactivity and Increased Systolic Blood Pressure Induced by the Placental Ischemia Model of Preeclampsia in Anesthetized Rats.Int J Mol Sci. 2023 Aug 15;24(16):12818. doi: 10.3390/ijms241612818. Int J Mol Sci. 2023. PMID: 37628999 Free PMC article.
-
Vasodilator Responses of Perivascular Adipose Tissue-Derived Hydrogen Sulfide Stimulated with L-Cysteine in Pregnancy Hypertension-Induced Endothelial Dysfunction in Rats.Antioxidants (Basel). 2023 Oct 26;12(11):1919. doi: 10.3390/antiox12111919. Antioxidants (Basel). 2023. PMID: 38001772 Free PMC article.
References
-
- The American College of Obstetricians and Gynecologists Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013;122:1122–1131. - PubMed
-
- Honigberg M.C., Riise H.K.R., Daltveit A.K., Tell G.S., Sulo G., Igland J., Klungsoyr K., Scott N.S., Wood M.J., Natarajan P., et al. Heart Failure in Women with Hypertensive Disorders of Pregnancy: Insights from the Cardiovascular Disease in Norway Project. Hypertension. 2020;76:1506–1513. doi: 10.1161/HYPERTENSIONAHA.120.15654. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

