The Role of RASGRP2 in Vascular Endothelial Cells-A Mini Review
- PMID: 34681791
- PMCID: PMC8537898
- DOI: 10.3390/ijms222011129
The Role of RASGRP2 in Vascular Endothelial Cells-A Mini Review
Abstract
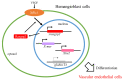
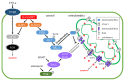
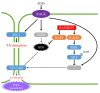
RAS guanyl nucleotide-releasing proteins (RASGRPs) are important proteins that act as guanine nucleotide exchange factors, which activate small GTPases and function as molecular switches for intracellular signals. The RASGRP family is composed of RASGRP1-4 proteins and activates the small GTPases, RAS and RAP. Among them, RASGRP2 has different characteristics from other RASGRPs in that it targets small GTPases and its localizations are different. Many studies related to RASGRP2 have been reported in cells of the blood cell lineage. Furthermore, RASGRP2 has also been reported to be associated with Huntington's disease, tumors, and rheumatoid arthritis. In addition, we also recently reported RASGRP2 expression in vascular endothelial cells, and clarified the involvement of xenopus Rasgrp2 in the vasculogenesis process and multiple signaling pathways of RASGRP2 in human vascular endothelial cells with stable expression of RASGRP2. Therefore, this article outlines the existing knowledge of RASGRP2 and focuses on its expression and role in vascular endothelial cells, and suggests that RASGRP2 functions as a protective factor for maintaining healthy blood vessels.
Keywords: R-RAS; RAP1; RAS guanyl nucleotide-releasing protein 2 (RASGRP2); small GTPase; vascular endothelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[Study on the Role of RASGRP2 in Vascular Endothelial Cells].Yakugaku Zasshi. 2023;143(11):917-922. doi: 10.1248/yakushi.23-00113. Yakugaku Zasshi. 2023. PMID: 37914339 Japanese.
-
Ras guanyl nucleotide releasing protein 2 affects cell viability and cell-matrix adhesion in ECV304 endothelial cells.Cell Adh Migr. 2013 May-Jun;7(3):262-6. doi: 10.4161/cam.24082. Epub 2013 Apr 5. Cell Adh Migr. 2013. PMID: 23563504 Free PMC article.
-
RASGRP2 Suppresses Apoptosis via Inhibition of ROS Production in Vascular Endothelial Cells.ScientificWorldJournal. 2019 Jan 1;2019:4639165. doi: 10.1155/2019/4639165. eCollection 2019. ScientificWorldJournal. 2019. PMID: 30692874 Free PMC article.
-
cAMP signalling in the vasculature: the role of Epac (exchange protein directly activated by cAMP).Biochem Soc Trans. 2014 Feb;42(1):89-97. doi: 10.1042/BST20130253. Biochem Soc Trans. 2014. PMID: 24450633 Review.
-
Rho guanine exchange factors in blood vessels: fine-tuners of angiogenesis and vascular function.Exp Cell Res. 2013 May 15;319(9):1289-97. doi: 10.1016/j.yexcr.2012.12.015. Epub 2012 Dec 21. Exp Cell Res. 2013. PMID: 23261542 Review.
Cited by
-
Rap1b: A cytoskeletal regulator Advantageous to viral infection.iScience. 2024 Sep 24;27(10):111023. doi: 10.1016/j.isci.2024.111023. eCollection 2024 Oct 18. iScience. 2024. PMID: 39474066 Free PMC article. Review.
-
Effectiveness of Kinesiotherapy in the Treatment of Achilles Tendinopathy-A Narrative Review.Sports (Basel). 2024 Jul 25;12(8):202. doi: 10.3390/sports12080202. Sports (Basel). 2024. PMID: 39195578 Free PMC article. Review.
-
Association between MAPK and PI3K/Akt signaling pathway-related gene polymorphisms and migraine.Mol Genet Genomic Med. 2024 Aug;12(8):e2503. doi: 10.1002/mgg3.2503. Mol Genet Genomic Med. 2024. PMID: 39140707 Free PMC article.
-
Identification of common molecular signatures of SARS-CoV-2 infection and its influence on acute kidney injury and chronic kidney disease.Front Immunol. 2023 Mar 21;14:961642. doi: 10.3389/fimmu.2023.961642. eCollection 2023. Front Immunol. 2023. PMID: 37026010 Free PMC article.
-
RASGRP2 is a potential immune-related biomarker and regulates mitochondrial-dependent apoptosis in lung adenocarcinoma.Front Immunol. 2023 Feb 3;14:1100231. doi: 10.3389/fimmu.2023.1100231. eCollection 2023. Front Immunol. 2023. PMID: 36817422 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

