Influence of Redox Imbalances on the Transposition of Insertion Sequences in Deinococcus geothermalis
- PMID: 34679757
- PMCID: PMC8533066
- DOI: 10.3390/antiox10101623
Influence of Redox Imbalances on the Transposition of Insertion Sequences in Deinococcus geothermalis
Abstract
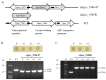
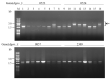
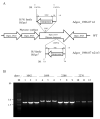
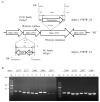
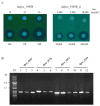
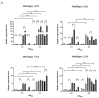
The transposition of insertion sequence elements was evaluated among different Deinococcus geothermalis lineages, including the wild-type, a cystine importer-disrupted mutant, a complemented strain, and a cystine importer-overexpressed strain. Cellular growth reached early exponential growth at OD600 2.0 and late exponential growth at OD600 4.0. Exposing the cells to hydrogen peroxide (80-100 mM) resulted in the transposition of insertion sequences (ISs) in genes associated with the carotenoid biosynthesis pathway. Particularly, ISDge7 (an IS5 family member) and ISDge5 (an IS701 family member) from the cystine importer-disrupted mutant were transposed into phytoene desaturase (dgeo_0524) via replicative transposition. Further, the cystine importer-overexpressed strain Δdgeo_1985R showed transposition of both ISDge2 and ISDge5 elements. In contrast, IS transposition was not detected in the complementary strain. Interestingly, a cystine importer-overexpressing strain exhibited streptomycin resistance, indicating that point mutation occurred in the rpsL (dgeo_1873) gene encoding ribosomal protein S12. qRT-PCR analyses were then conducted to evaluate the expression of oxidative stress response genes, IS elements, and low-molecular-weight thiol compounds such as mycothiol and bacillithiol. Nevertheless, the mechanisms that trigger IS transposition in redox imbalance conditions remain unclear. Here, we report that the active transposition of different IS elements was affected by intracellular redox imbalances caused by cystine importer deficiencies or overexpression.
Keywords: Deinococcus geothermalis; cystine importer; insertion sequences; oxidative stress; redox-balance; transcriptomic analysis; transposition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Active Transposition of Insertion Sequences in Prokaryotes: Insights from the Response of Deinococcus geothermalis to Oxidative Stress.Antioxidants (Basel). 2022 Feb 28;11(3):481. doi: 10.3390/antiox11030481. Antioxidants (Basel). 2022. PMID: 35326130 Free PMC article. Review.
-
The Transposition of Insertion Sequences in Sigma-Factor- and LysR-Deficient Mutants of Deinococcus geothermalis.Microorganisms. 2024 Feb 4;12(2):328. doi: 10.3390/microorganisms12020328. Microorganisms. 2024. PMID: 38399731 Free PMC article.
-
Transposition of insertion sequences by dielectric barrier discharge plasma and gamma irradiation in the radiation-resistant bacterium Deinococcus geothermalis.J Microbiol Methods. 2022 May;196:106473. doi: 10.1016/j.mimet.2022.106473. Epub 2022 Apr 22. J Microbiol Methods. 2022. PMID: 35469976
-
Redox potential change by the cystine importer affected on enzymatic antioxidant protection in Deinococcus geothermalis.Antonie Van Leeuwenhoek. 2020 Jun;113(6):779-790. doi: 10.1007/s10482-020-01388-4. Epub 2020 Jan 28. Antonie Van Leeuwenhoek. 2020. PMID: 31993844
-
The IS200/IS605 Family and "Peel and Paste" Single-strand Transposition Mechanism.Microbiol Spectr. 2015 Aug;3(4). doi: 10.1128/microbiolspec.MDNA3-0039-2014. Microbiol Spectr. 2015. PMID: 26350330 Review.
Cited by
-
Hydrogen peroxide treatment induces the transposition of an insertion sequence in Deinococcus radiopugnans DY59.Front Microbiol. 2023 Mar 2;14:1110084. doi: 10.3389/fmicb.2023.1110084. eCollection 2023. Front Microbiol. 2023. PMID: 36937269 Free PMC article.
-
Adaptive Response of Thermophiles to Redox Stress and Their Role in the Process of dye Degradation From Textile Industry Wastewater.Front Physiol. 2022 Jun 20;13:908370. doi: 10.3389/fphys.2022.908370. eCollection 2022. Front Physiol. 2022. PMID: 35795652 Free PMC article. Review.
-
Active Transposition of Insertion Sequences in Prokaryotes: Insights from the Response of Deinococcus geothermalis to Oxidative Stress.Antioxidants (Basel). 2022 Feb 28;11(3):481. doi: 10.3390/antiox11030481. Antioxidants (Basel). 2022. PMID: 35326130 Free PMC article. Review.
-
The Transposition of Insertion Sequences in Sigma-Factor- and LysR-Deficient Mutants of Deinococcus geothermalis.Microorganisms. 2024 Feb 4;12(2):328. doi: 10.3390/microorganisms12020328. Microorganisms. 2024. PMID: 38399731 Free PMC article.
References
-
- Ohtsu I., Kawano Y., Suzuki M., Morigasaki S., Saiki K., Yamazaki S., Nonaka G., Takagi H. Uptake of L-cystine via an ABC transporter contributes defense of oxidative stress in the L-cystine export-dependent manner in Escherichia coli. PLoS ONE. 2015;10:e0120619. doi: 10.1371/journal.pone.0120619. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

