Regulator of Cell Cycle Protein (RGCC/RGC-32) Protects against Pulmonary Fibrosis
- PMID: 34668840
- PMCID: PMC8845131
- DOI: 10.1165/rcmb.2021-0022OC
Regulator of Cell Cycle Protein (RGCC/RGC-32) Protects against Pulmonary Fibrosis
Abstract
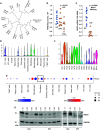
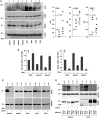
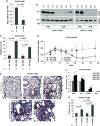
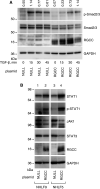
Some previous studies in tissue fibrosis have suggested a profibrotic contribution from elevated expression of a protein termed either RGCC (regulator of cell cycle) or RGC-32 (response gene to complement 32 protein). Our analysis of public gene expression datasets, by contrast, revealed a consistent decrease in RGCC mRNA levels in association with pulmonary fibrosis. Consistent with this observation, we found that stimulating primary adult human lung fibroblasts with transforming growth factor (TGF)-β in cell cultures elevated collagen expression and simultaneously attenuated RGCC mRNA and protein levels. Moreover, overexpression of RGCC in cultured lung fibroblasts attenuated the stimulating effect of TGF-β on collagen levels. Similar to humans with pulmonary fibrosis, the levels of RGCC were also decreased in vivo in lung tissues of wild-type mice challenged with bleomycin in both acute and chronic models. Mice with constitutive RGCC gene deletion accumulated more collagen in their lungs in response to chronic bleomycin challenge than did wild-type mice. RNA-Seq analyses of lung fibroblasts revealed that RGCC overexpression alone had a modest transcriptomic effect, but in combination with TGF-β stimulation, induced notable transcriptomic changes that negated the effects of TGF-β, including on extracellular matrix-related genes. At the level of intracellular signaling, RGCC overexpression delayed early TGF-β-induced Smad2/3 phosphorylation, elevated the expression of total and phosphorylated antifibrotic mediator STAT1, and attenuated the expression of a profibrotic mediator STAT3. We conclude that RGCC plays a protective role in pulmonary fibrosis and that its decline permits collagen accumulation. Restoration of RGCC expression may have therapeutic potential in pulmonary fibrosis.
Keywords: idiopathic pulmonary fibrosis; lung fibrosis; regulator of cell cycle protein; response gene to complement 32 protein; scleroderma lung disease.
Figures

Comment in
-
Pumping the Brakes on Pulmonary Fibrosis: A New Role for Regulator of Cell Cycle.Am J Respir Cell Mol Biol. 2022 Feb;66(2):113-114. doi: 10.1165/rcmb.2021-0399ED. Am J Respir Cell Mol Biol. 2022. PMID: 34758280 Free PMC article. No abstract available.
Similar articles
-
P311 Promotes Lung Fibrosis via Stimulation of Transforming Growth Factor-β1, -β2, and -β3 Translation.Am J Respir Cell Mol Biol. 2019 Feb;60(2):221-231. doi: 10.1165/rcmb.2018-0028OC. Am J Respir Cell Mol Biol. 2019. PMID: 30230348 Free PMC article.
-
The inhibitory effect of ginsan on TGF-β mediated fibrotic process.J Cell Physiol. 2011 May;226(5):1241-7. doi: 10.1002/jcp.22452. J Cell Physiol. 2011. PMID: 20945375
-
EM703 improves bleomycin-induced pulmonary fibrosis in mice by the inhibition of TGF-beta signaling in lung fibroblasts.Respir Res. 2006 Jan 27;7(1):16. doi: 10.1186/1465-9921-7-16. Respir Res. 2006. PMID: 16438734 Free PMC article.
-
Lysocardiolipin acyltransferase regulates TGF-β mediated lung fibroblast differentiation.Free Radic Biol Med. 2017 Nov;112:162-173. doi: 10.1016/j.freeradbiomed.2017.07.023. Epub 2017 Jul 24. Free Radic Biol Med. 2017. PMID: 28751023
-
A long-acting isomer of Ac-SDKP attenuates pulmonary fibrosis through SRPK1-mediated PI3K/AKT and Smad2 pathway inhibition.IUBMB Life. 2020 Dec;72(12):2611-2626. doi: 10.1002/iub.2389. Epub 2020 Nov 2. IUBMB Life. 2020. PMID: 33135306
Cited by
-
Single-Cell RNA-Sequencing Analyses Identify APLNR, INS-IGF2, RGCC Genes May Be Involved in the Pathogenesis of Systemic Sclerosis Skin.Clin Cosmet Investig Dermatol. 2024 May 9;17:1059-1069. doi: 10.2147/CCID.S456593. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 38742168 Free PMC article.
-
Novel insight into the underlying dysregulation mechanisms of immune cell-to-cell communication by analyzing multitissue single-cell atlas of two COVID-19 patients.Cell Death Dis. 2023 Apr 22;14(4):286. doi: 10.1038/s41419-023-05814-z. Cell Death Dis. 2023. PMID: 37087411 Free PMC article.
-
Full-length IL-33 augments pulmonary fibrosis in an ST2- and Th2-independent, non-transcriptomic fashion.Cell Immunol. 2023 Jan;383:104657. doi: 10.1016/j.cellimm.2022.104657. Epub 2022 Dec 16. Cell Immunol. 2023. PMID: 36603504 Free PMC article.
-
Effect of Semaglutide and Empagliflozin on Pulmonary Structure and Proteomics in Obese Mice.Diabetes Metab Syndr Obes. 2024 Mar 11;17:1217-1233. doi: 10.2147/DMSO.S456336. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38496002 Free PMC article.
-
Pumping the Brakes on Pulmonary Fibrosis: A New Role for Regulator of Cell Cycle.Am J Respir Cell Mol Biol. 2022 Feb;66(2):113-114. doi: 10.1165/rcmb.2021-0399ED. Am J Respir Cell Mol Biol. 2022. PMID: 34758280 Free PMC article. No abstract available.
References
-
- Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med . 2018;378:1811–1823. - PubMed
-
- Dempsey TM, Payne S, Sangaralingham L, Yao X, Shah ND, Limper AH. Adoption of the antifibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc . 2021;18:1121–1128. - PubMed
-
- Di Martino E, Provenzani A, Vitulo P, Polidori P. Systematic review and meta-analysis of pirfenidone, nintedanib, and pamrevlumab for the treatment of idiopathic pulmonary fibrosis. Ann Pharmacother . 2021;55:723–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

