Spatial transcriptomics reveals a role for sensory nerves in preserving cranial suture patency through modulation of BMP/TGF-β signaling
- PMID: 34663698
- PMCID: PMC8545472
- DOI: 10.1073/pnas.2103087118
Spatial transcriptomics reveals a role for sensory nerves in preserving cranial suture patency through modulation of BMP/TGF-β signaling
Abstract
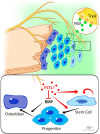
The patterning and ossification of the mammalian skeleton requires the coordinated actions of both intrinsic bone morphogens and extrinsic neurovascular signals, which function in a temporal and spatial fashion to control mesenchymal progenitor cell (MPC) fate. Here, we show the genetic inhibition of tropomyosin receptor kinase A (TrkA) sensory nerve innervation of the developing cranium results in premature calvarial suture closure, associated with a decrease in suture MPC proliferation and increased mineralization. In vitro, axons from peripheral afferent neurons derived from dorsal root ganglions (DRGs) of wild-type mice induce MPC proliferation in a spatially restricted manner via a soluble factor when cocultured in microfluidic chambers. Comparative spatial transcriptomic analysis of the cranial sutures in vivo confirmed a positive association between sensory axons and proliferative MPCs. SpatialTime analysis across the developing suture revealed regional-specific alterations in bone morphogenetic protein (BMP) and TGF-β signaling pathway transcripts in response to TrkA inhibition. RNA sequencing of DRG cell bodies, following direct, axonal coculture with MPCs, confirmed the alterations in BMP/TGF-β signaling pathway transcripts. Among these, the BMP inhibitor follistatin-like 1 (FSTL1) replicated key features of the neural-to-bone influence, including mitogenic and anti-osteogenic effects via the inhibition of BMP/TGF-β signaling. Taken together, our results demonstrate that sensory nerve-derived signals, including FSTL1, function to coordinate cranial bone patterning by regulating MPC proliferation and differentiation in the suture mesenchyme.
Keywords: TrkA; calvarial bone; cranial suture; skeletal innervation; spatial transcriptomics.
Conflict of interest statement
Competing interest statement: 10X Genomics provided supplies and expert consultation for the study. A.W.J. is a paid consultant for Novadip and Lifesprout LLC. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies.
Figures

Similar articles
-
Regional differentiation of cranial suture-associated dura mater in vivo and in vitro: implications for suture fusion and patency.J Bone Miner Res. 2000 Dec;15(12):2413-30. doi: 10.1359/jbmr.2000.15.12.2413. J Bone Miner Res. 2000. PMID: 11127206
-
Mechanisms of murine cranial suture patency mediated by a dominant negative transforming growth factor-beta receptor adenovirus.Plast Reconstr Surg. 2004 May;113(6):1685-97. doi: 10.1097/01.prs.0000117363.43699.5b. Plast Reconstr Surg. 2004. PMID: 15114130
-
TGF-beta1, FGF-2, and receptor mRNA expression in suture mesenchyme and dura versus underlying brain in fusing and nonfusing mouse cranial sutures.Plast Reconstr Surg. 2004 May;113(6):1675-84. doi: 10.1097/01.prs.0000117362.33347.43. Plast Reconstr Surg. 2004. PMID: 15114129
-
TGF-β and BMP signaling in osteoblast differentiation and bone formation.Int J Biol Sci. 2012;8(2):272-88. doi: 10.7150/ijbs.2929. Epub 2012 Jan 21. Int J Biol Sci. 2012. PMID: 22298955 Free PMC article. Review.
-
Apoptosis in membranous bone formation: role of fibroblast growth factor and bone morphogenetic protein signaling.Crit Rev Eukaryot Gene Expr. 2005;15(1):75-92. doi: 10.1615/critreveukaryotgeneexpr.v15.i1.50. Crit Rev Eukaryot Gene Expr. 2005. PMID: 15831079 Review.
Cited by
-
STimage-1K4M: A histopathology image-gene expression dataset for spatial transcriptomics.ArXiv [Preprint]. 2024 Jun 20:arXiv:2406.06393v2. ArXiv. 2024. PMID: 38947920 Free PMC article. Preprint.
-
The Role of Neuromodulation and Potential Mechanism in Regulating Heterotopic Ossification.Neurochem Res. 2024 Jul;49(7):1628-1642. doi: 10.1007/s11064-024-04118-8. Epub 2024 Feb 28. Neurochem Res. 2024. PMID: 38416374 Review.
-
Enhanced osteoblastic differentiation of parietal bone in a novel murine model of mucopolysaccharidosis type II.Mol Genet Metab Rep. 2023 Nov 11;37:101021. doi: 10.1016/j.ymgmr.2023.101021. eCollection 2023 Dec. Mol Genet Metab Rep. 2023. PMID: 38053930 Free PMC article.
-
The role of DNA methylation on gene expression in the vertebrae of ancestrally benzo[a]pyrene exposed F1 and F3 male medaka.Epigenetics. 2023 Dec;18(1):2222246. doi: 10.1080/15592294.2023.2222246. Epigenetics. 2023. PMID: 37322851 Free PMC article.
-
Spatial transcriptomics implicates impaired BMP signaling in NF1 fracture pseudarthrosis in murine and patient tissues.JCI Insight. 2024 Jul 11;9(16):e176802. doi: 10.1172/jci.insight.176802. JCI Insight. 2024. PMID: 38990653 Free PMC article.
References
-
- Lenton K. A., Nacamuli R. P., Wan D. C., Helms J. A., Longaker M. T., Cranial suture biology. Curr. Top. Dev. Biol. 66, 287–328 (2005). - PubMed
-
- Jiang X., Iseki S., Maxson R. E., Sucov H. M., Morriss-Kay G. M., Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 241, 106–116 (2002). - PubMed
-
- Bellus G. A., et al. ., Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nat. Genet. 14, 174–176 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

