The regulation of actin dynamics during cell division and malignancy
- PMID: 34659876
- PMCID: PMC8493394
The regulation of actin dynamics during cell division and malignancy
Abstract
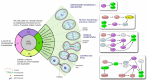
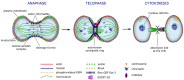
Actin is the most abundant protein in almost all the eukaryotic cells. Actin amino acid sequences are highly conserved and have not changed a lot during the progress of evolution, varying by no more than 20% in the completely different species, such as humans and algae. The network of actin filaments plays a crucial role in regulating cells' cytoskeleton that needs to undergo dynamic tuning and structural changes in order for various functional processes, such as cell motility, migration, adhesion, polarity establishment, cell growth and cell division, to take place in live cells. Owing to its fundamental role in the cell, actin is a prominent regulator of cell division, a process, whose success directly depends on morphological changes of actin cytoskeleton and correct segregation of duplicated chromosomes. Disorganization of actin framework during the last stage of cell division, known as cytokinesis, can lead to multinucleation and formation of polyploidy in post-mitotic cells, eventually developing into cancer. In this review, we will cover the principles of actin regulation during cell division and discuss how the control of actin dynamics is altered during the state of malignancy.
Keywords: Actin; abscission; cancer; cleavage furrow; contractile ring; cytokinesis; mitosis; myosin.
AJCR Copyright © 2021.
Conflict of interest statement
None.
Figures

Similar articles
-
Actomyosin organization during cytokinesis: reversible translocation and differential redistribution in Dictyostelium.Cell Motil Cytoskeleton. 1989;12(2):78-89. doi: 10.1002/cm.970120203. Cell Motil Cytoskeleton. 1989. PMID: 2713900
-
Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells.Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. Epub 2014 Jul 2. Small GTPases. 2014. PMID: 24988197 Free PMC article. Review.
-
Regulation of the actin cytoskeleton by PIP2 in cytokinesis.Biol Cell. 2006 Jun;98(6):377-88. doi: 10.1042/BC20050081. Biol Cell. 2006. PMID: 16704377 Review.
-
[Rho proteins - the key regulators of cytoskeleton in the progression of mitosis and cytokinesis].Postepy Hig Med Dosw (Online). 2011 Nov 18;65:704-13. doi: 10.5604/17322693.966197. Postepy Hig Med Dosw (Online). 2011. PMID: 22100803 Review. Polish.
-
Mechanism of the formation of contractile ring in dividing cultured animal cells. I. Recruitment of preexisting actin filaments into the cleavage furrow.J Cell Biol. 1990 Apr;110(4):1089-95. doi: 10.1083/jcb.110.4.1089. J Cell Biol. 1990. PMID: 2324193 Free PMC article.
Cited by
-
Proteomic Analysis Reveals Major Proteins and Pathways That Mediate the Effect of 17-β-Estradiol in Cell Division and Apoptosis in Breast Cancer MCF7 Cells.J Proteome Res. 2024 Nov 1;23(11):4835-4848. doi: 10.1021/acs.jproteome.4c00102. Epub 2024 Oct 11. J Proteome Res. 2024. PMID: 39392593 Free PMC article.
-
The Molecular Mechanisms Employed by the Parasite Myxobolus bejeranoi (Cnidaria: Myxozoa) from Invasion through Sporulation for Successful Proliferation in Its Fish Host.Int J Mol Sci. 2023 Aug 15;24(16):12824. doi: 10.3390/ijms241612824. Int J Mol Sci. 2023. PMID: 37629003 Free PMC article.
-
Claudin-4 remodeling of nucleus-cell cycle crosstalk maintains ovarian tumor genome stability and drives resistance to genomic instability-inducing agents.bioRxiv [Preprint]. 2024 Sep 7:2024.09.04.611120. doi: 10.1101/2024.09.04.611120. bioRxiv. 2024. PMID: 39282307 Free PMC article. Preprint.
-
Transcriptomics reveal useful resources for examining fruit development and variation in fruit size in Coccinia grandis.Front Plant Sci. 2024 May 28;15:1386041. doi: 10.3389/fpls.2024.1386041. eCollection 2024. Front Plant Sci. 2024. PMID: 38863541 Free PMC article.
-
The Actin Regulators Involved in the Function and Related Diseases of Lymphocytes.Front Immunol. 2022 Mar 16;13:799309. doi: 10.3389/fimmu.2022.799309. eCollection 2022. Front Immunol. 2022. PMID: 35371070 Free PMC article. Review.
References
-
- Herman IM. Actin isoforms. Curr Opin Cell Biol. 1993;5:48–55. - PubMed
-
- Rubenstein PA. The functional importance of multiple actin isoforms. BioEssays. 1990;12:309–315. - PubMed
-
- Dugina VB, Shagieva GS, Kopnin PB. Biological role of actin isoforms in mammalian cells. Biochemistry (Mosc) 2019;84:583–592. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
