Eosinophils Suppress the Migration of T Cells Into the Brain of Plasmodium berghei-Infected Ifnar1-/- Mice and Protect Them From Experimental Cerebral Malaria
- PMID: 34659202
- PMCID: PMC8514736
- DOI: 10.3389/fimmu.2021.711876
Eosinophils Suppress the Migration of T Cells Into the Brain of Plasmodium berghei-Infected Ifnar1-/- Mice and Protect Them From Experimental Cerebral Malaria
Abstract
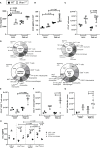
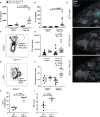
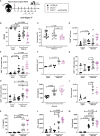
Cerebral malaria is a potentially lethal disease, which is caused by excessive inflammatory responses to Plasmodium parasites. Here we use a newly developed transgenic Plasmodium berghei ANKA (PbAAma1OVA) parasite that can be used to study parasite-specific T cell responses. Our present study demonstrates that Ifnar1-/- mice, which lack type I interferon receptor-dependent signaling, are protected from experimental cerebral malaria (ECM) when infected with this novel parasite. Although CD8+ T cell responses generated in the spleen are essential for the development of ECM, we measured comparable parasite-specific cytotoxic T cell responses in ECM-protected Ifnar1-/- mice and wild type mice suffering from ECM. Importantly, CD8+ T cells were increased in the spleens of ECM-protected Ifnar1-/- mice and the blood-brain-barrier remained intact. This was associated with elevated splenic levels of CCL5, a T cell and eosinophil chemotactic chemokine, which was mainly produced by eosinophils, and an increase in eosinophil numbers. Depletion of eosinophils enhanced CD8+ T cell infiltration into the brain and increased ECM induction in PbAAma1OVA-infected Ifnar1-/- mice. However, eosinophil-depletion did not reduce the CD8+ T cell population in the spleen or reduce splenic CCL5 concentrations. Our study demonstrates that eosinophils impact CD8+ T cell migration and proliferation during PbAAma1OVA-infection in Ifnar1-/- mice and thereby are contributing to the protection from ECM.
Keywords: CCL5; CD8 T cells; IFNAR1; NK cells; Plasmodium berghei; eosinophils; experimental cerebral malaria (ECM); malaria.
Copyright © 2021 Scheunemann, Reichwald, Korir, Kuehlwein, Jenster, Hammerschmidt-Kamper, Lewis, Klocke, Borsche, Schwendt, Soun, Thiebes, Limmer, Engel, Mueller, Hoerauf, Hübner and Schumak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
IFNAR1 controls progression to cerebral malaria in children and CD8+ T cell brain pathology in Plasmodium berghei-infected mice.J Immunol. 2013 May 15;190(10):5118-27. doi: 10.4049/jimmunol.1300114. Epub 2013 Apr 12. J Immunol. 2013. PMID: 23585679
-
Type I interferons contribute to experimental cerebral malaria development in response to sporozoite or blood-stage Plasmodium berghei ANKA.Eur J Immunol. 2013 Oct;43(10):2683-95. doi: 10.1002/eji.201343327. Epub 2013 Jul 19. Eur J Immunol. 2013. PMID: 23780878
-
The transcription factor T-bet regulates parasitemia and promotes pathogenesis during Plasmodium berghei ANKA murine malaria.J Immunol. 2013 Nov 1;191(9):4699-708. doi: 10.4049/jimmunol.1300396. Epub 2013 Sep 27. J Immunol. 2013. PMID: 24078698
-
Protection of Batf3-deficient mice from experimental cerebral malaria correlates with impaired cytotoxic T-cell responses and immune regulation.Immunology. 2020 Feb;159(2):193-204. doi: 10.1111/imm.13137. Epub 2019 Nov 13. Immunology. 2020. PMID: 31631339 Free PMC article.
-
Pathogenic CD8+ T cells in experimental cerebral malaria.Semin Immunopathol. 2015 May;37(3):221-31. doi: 10.1007/s00281-015-0476-6. Epub 2015 Mar 13. Semin Immunopathol. 2015. PMID: 25772948 Review.
References
-
- World Health Organization . World Malaria Report 2019. [S.l.]. Geneva: World Health Organization; (2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

