Lysophosphatidic Acid-Induced EGFR Transactivation Promotes Gastric Cancer Cell DNA Replication by Stabilizing Geminin in the S Phase
- PMID: 34658851
- PMCID: PMC8511314
- DOI: 10.3389/fphar.2021.706240
Lysophosphatidic Acid-Induced EGFR Transactivation Promotes Gastric Cancer Cell DNA Replication by Stabilizing Geminin in the S Phase
Abstract
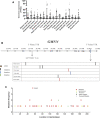
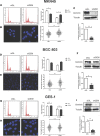
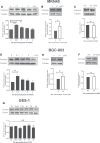
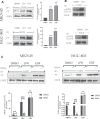
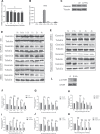
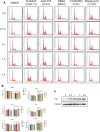
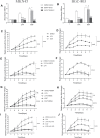
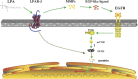
Geminin, an inhibitor of the DNA replication licensing factor, chromatin licensing and DNA replication factor (Cdt) 1, is essential for the maintenance of genomic integrity. As a multifunctional protein, geminin is also involved in tumor progression, but the molecular details are largely unknown. Here, we found that lysophosphatidic acid (LPA)-induced upregulation of geminin was specific to gastric cancer cells. LPA acted via LPA receptor (LPAR) 3 and matrix metalloproteinases (MMPs) signaling to transactivate epidermal growth factor receptor (EGFR) (Y1173) and thereby stabilize geminin expression level during the S phase. LPA also induced the expression of deubiquitinating protein (DUB) 3, which prevented geminin degradation. These results reveal a novel mechanism underlying gastric cancer progression that involves the regulation of geminin stability by LPA-induced EGFR transactivation and provide potential targets for the signaling pathway and tumor cell-specific inhibitors.
Keywords: DNA replication; EGFR; LPA; geminin; transactivation.
Copyright © 2021 Zhao, Gezi, Tian, Jia, Morigen and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Lysophosphatidic acid suppresses apoptosis of high-grade serous ovarian cancer cells by inducing autophagy activity and promotes cell-cycle progression via EGFR-PI3K/Aurora-AThr288-geminin dual signaling pathways.Front Pharmacol. 2022 Dec 19;13:1046269. doi: 10.3389/fphar.2022.1046269. eCollection 2022. Front Pharmacol. 2022. PMID: 36601056 Free PMC article.
-
Lysophosphatidic acid induces both EGFR-dependent and EGFR-independent effects on DNA synthesis and migration in pancreatic and colorectal carcinoma cells.Tumour Biol. 2016 Feb;37(2):2519-26. doi: 10.1007/s13277-015-4010-1. Epub 2015 Sep 19. Tumour Biol. 2016. PMID: 26386720
-
TNF-α and LPA promote synergistic expression of COX-2 in human colonic myofibroblasts: role of LPA-mediated transactivation of upregulated EGFR.BMC Gastroenterol. 2013 May 20;13:90. doi: 10.1186/1471-230X-13-90. BMC Gastroenterol. 2013. PMID: 23688423 Free PMC article.
-
GPR87 mediates lysophosphatidic acid-induced colony dispersal in A431 cells.Eur J Pharmacol. 2013 Sep 5;715(1-3):15-20. doi: 10.1016/j.ejphar.2013.06.029. Epub 2013 Jul 4. Eur J Pharmacol. 2013. PMID: 23831392 Review.
-
Geminin a multi task protein involved in cancer pathophysiology and developmental process: A review.Biochimie. 2016 Dec;131:115-127. doi: 10.1016/j.biochi.2016.09.022. Epub 2016 Oct 1. Biochimie. 2016. PMID: 27702582 Review.
Cited by
-
Lysophosphatidic Acid Signaling in the Gastrointestinal System.Cell Mol Gastroenterol Hepatol. 2024;18(6):101398. doi: 10.1016/j.jcmgh.2024.101398. Epub 2024 Sep 2. Cell Mol Gastroenterol Hepatol. 2024. PMID: 39233124 Free PMC article. Review.
-
The Emerging Role of LPA as an Oncometabolite.Cells. 2024 Apr 4;13(7):629. doi: 10.3390/cells13070629. Cells. 2024. PMID: 38607068 Free PMC article. Review.
-
Lysophosphatidic acid suppresses apoptosis of high-grade serous ovarian cancer cells by inducing autophagy activity and promotes cell-cycle progression via EGFR-PI3K/Aurora-AThr288-geminin dual signaling pathways.Front Pharmacol. 2022 Dec 19;13:1046269. doi: 10.3389/fphar.2022.1046269. eCollection 2022. Front Pharmacol. 2022. PMID: 36601056 Free PMC article.
References
-
- Alsahafi E. N., Thavaraj S., Sarvestani N., Novoplansky O., Elkabets M., Ayaz B., et al. (2020). EGFR Overexpression Increases Radiotherapy Response in HPV-Positive Head and Neck Cancer through Inhibition of DNA Damage Repair and HPV E6 Downregulation. Cancer Lett. 498, 80–97. 10.1016/j.canlet.2020.10.035 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

