Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes
- PMID: 34658302
- PMCID: PMC8865583
- DOI: 10.1164/rccm.202109-2205PP
Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes
Abstract
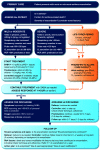
The Global Initiative for Asthma (GINA) Strategy Report provides clinicians with an annually updated evidence-based strategy for asthma management and prevention, which can be adapted for local circumstances (e.g., medication availability). This article summarizes key recommendations from GINA 2021, and the evidence underpinning recent changes. GINA recommends that asthma in adults and adolescents should not be treated solely with short-acting β2-agonist (SABA), because of the risks of SABA-only treatment and SABA overuse, and evidence for benefit of inhaled corticosteroids (ICS). Large trials show that as-needed combination ICS-formoterol reduces severe exacerbations by ⩾60% in mild asthma compared with SABA alone, with similar exacerbation, symptom, lung function, and inflammatory outcomes as daily ICS plus as-needed SABA. Key changes in GINA 2021 include division of the treatment figure for adults and adolescents into two tracks. Track 1 (preferred) has low-dose ICS-formoterol as the reliever at all steps: as needed only in Steps 1-2 (mild asthma), and with daily maintenance ICS-formoterol (maintenance-and-reliever therapy, "MART") in Steps 3-5. Track 2 (alternative) has as-needed SABA across all steps, plus regular ICS (Step 2) or ICS-long-acting β2-agonist (Steps 3-5). For adults with moderate-to-severe asthma, GINA makes additional recommendations in Step 5 for add-on long-acting muscarinic antagonists and azithromycin, with add-on biologic therapies for severe asthma. For children 6-11 years, new treatment options are added at Steps 3-4. Across all age groups and levels of severity, regular personalized assessment, treatment of modifiable risk factors, self-management education, skills training, appropriate medication adjustment, and review remain essential to optimize asthma outcomes.
Keywords: asthma; asthma diagnosis; asthma management; asthma prevention.
Figures





Comment in
-
Global Initiative for Asthma 2021: Asthma in Preschool Children and Short-Acting β2-Agonist-Only Treatment.Am J Respir Crit Care Med. 2022 Apr 15;205(8):971-972. doi: 10.1164/rccm.202111-2465LE. Am J Respir Crit Care Med. 2022. PMID: 35202554 Free PMC article. No abstract available.
Similar articles
-
Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes.Respirology. 2022 Jan;27(1):14-35. doi: 10.1111/resp.14174. Respirology. 2022. PMID: 34668278
-
Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes.J Allergy Clin Immunol Pract. 2022 Jan;10(1S):S1-S18. doi: 10.1016/j.jaip.2021.10.001. Epub 2021 Oct 28. J Allergy Clin Immunol Pract. 2022. PMID: 34718211
-
Global Initiative for Asthma Strategy 2021. Executive Summary and Rationale for Key Changes.Arch Bronconeumol. 2022 Jan;58(1):35-51. doi: 10.1016/j.arbres.2021.10.003. Epub 2021 Oct 28. Arch Bronconeumol. 2022. PMID: 35245179 English, Spanish.
-
Symbicort® Maintenance and Reliever Therapy (SMART) and the evolution of asthma management within the GINA guidelines.Expert Rev Respir Med. 2018 Mar;12(3):191-202. doi: 10.1080/17476348.2018.1429921. Epub 2018 Feb 5. Expert Rev Respir Med. 2018. PMID: 29400090 Review.
-
The Role of ICS-Containing Rescue Therapy Versus SABA Alone in Asthma Management Today.J Allergy Clin Immunol Pract. 2024 Apr;12(4):870-879. doi: 10.1016/j.jaip.2024.01.011. Epub 2024 Jan 17. J Allergy Clin Immunol Pract. 2024. PMID: 38237858 Review.
Cited by
-
The production, function, and clinical applications of IL-33 in type 2 inflammation-related respiratory diseases.Front Immunol. 2024 Sep 5;15:1436437. doi: 10.3389/fimmu.2024.1436437. eCollection 2024. Front Immunol. 2024. PMID: 39301028 Free PMC article. Review.
-
Household income unequally affects genetic susceptibility to pulmonary diseases: evidence from bidirectional Mendelian randomization study.Front Med (Lausanne). 2024 Jul 4;11:1279697. doi: 10.3389/fmed.2024.1279697. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39026555 Free PMC article.
-
Comprehensive analysis of immune-related genes for classification and immune microenvironment of asthma.Am J Transl Res. 2023 Feb 15;15(2):1052-1062. eCollection 2023. Am J Transl Res. 2023. PMID: 36915798 Free PMC article.
-
Long COVID outcomes in an asthmatic cohort and its implications for asthma control.Respir Med. 2023 Feb;207:107092. doi: 10.1016/j.rmed.2022.107092. Epub 2022 Dec 16. Respir Med. 2023. PMID: 36535372 Free PMC article. No abstract available.
-
An Overview of the Obese-Asthma Phenotype in Children.Int J Environ Res Public Health. 2022 Jan 6;19(2):636. doi: 10.3390/ijerph19020636. Int J Environ Res Public Health. 2022. PMID: 35055456 Free PMC article. Review.
References
-
- Global Asthma Network. The global asthma report 2018 Auckland, New Zealand: Global Asthma Network; 2018. [accessed 2021 Sep]. Available from: http://globalasthmareport.org
-
- National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program; World Health Organization; National Institutes of Health Publication No. 95-3659. Global Initiative for Asthma. Bethesda, MD: National Institutes of Health; 1995.
-
- Global Initiative for Asthma Methodology. Fontana, WI: Global Initiative for Asthma; 2021. [accessed 2021 Sep]. Available from: https://ginasthma.org/about-us/methodology
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention Fontana, WI: Global Initiative for Asthma; 2021. [accessed 2021 Sep]. Available from: https://www.ginasthma.org/reports
-
- Aaron SD, Vandemheen KL, FitzGerald JM, Ainslie M, Gupta S, Lemière C, et al. Canadian Respiratory Research Network Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA . 2017;317:269–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

