The penalty of stress - Epichaperomes negatively reshaping the brain in neurodegenerative disorders
- PMID: 34657288
- PMCID: PMC8688321
- DOI: 10.1111/jnc.15525
The penalty of stress - Epichaperomes negatively reshaping the brain in neurodegenerative disorders
Abstract
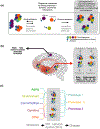
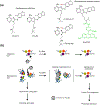
Adaptation to acute and chronic stress and/or persistent stressors is a subject of wide interest in central nervous system disorders. In this context, stress is an effector of change in organismal homeostasis and the response is generated when the brain perceives a potential threat. Herein, we discuss a nuanced and granular view whereby a wide variety of genotoxic and environmental stressors, including aging, genetic risk factors, environmental exposures, and age- and lifestyle-related changes, act as direct insults to cellular, as opposed to organismal, homeostasis. These two concepts of how stressors impact the central nervous system are not mutually exclusive. We discuss how maladaptive stressor-induced changes in protein connectivity through epichaperomes, disease-associated pathologic scaffolds composed of tightly bound chaperones, co-chaperones, and other factors, impact intracellular protein functionality altering phenotypes, that in turn disrupt and remodel brain networks ranging from intercellular to brain connectome levels. We provide an evidence-based view on how these maladaptive changes ranging from stressor to phenotype provide unique precision medicine opportunities for diagnostic and therapeutic development, especially in the context of neurodegenerative disorders including Alzheimer's disease where treatment options are currently limited.
Keywords: Alzheimer's disease; chronic stress and stressors; epichaperomes; maladaptive response to stress; stressor-to-phenotype; synaptic plasticity.
© 2021 International Society for Neurochemistry.
Conflict of interest statement
CONFLICT OF INTEREST
Memorial Sloan Kettering Cancer Center holds the intellectual rights to PU-H71 and PU-AD. Samus Therapeutics Inc, of which G.C. has partial ownership, and is a member of its board of directors, has licensed this portfolio. G.C. is an inventor on the licensed intellectual property. All other authors declare no competing interests.
Figures

Similar articles
-
The effect of aged microglia on synaptic impairment and its relevance in neurodegenerative diseases.Neurochem Int. 2021 Mar;144:104982. doi: 10.1016/j.neuint.2021.104982. Epub 2021 Feb 5. Neurochem Int. 2021. PMID: 33556444 Review.
-
Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach.Amino Acids. 2003 Dec;25(3-4):437-44. doi: 10.1007/s00726-003-0048-2. Epub 2003 Nov 7. Amino Acids. 2003. PMID: 14661103 Review.
-
NAD+ in Brain Aging and Neurodegenerative Disorders.Cell Metab. 2019 Oct 1;30(4):630-655. doi: 10.1016/j.cmet.2019.09.001. Cell Metab. 2019. PMID: 31577933 Free PMC article. Review.
-
Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders.Neuromolecular Med. 2002;2(2):215-31. doi: 10.1385/NMM:2:2:215. Neuromolecular Med. 2002. PMID: 12428812 Review.
-
Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
Cited by
-
Introducing dysfunctional Protein-Protein Interactome (dfPPI) - A platform for systems-level protein-protein interaction (PPI) dysfunction investigation in disease.Curr Opin Struct Biol. 2024 Oct;88:102886. doi: 10.1016/j.sbi.2024.102886. Epub 2024 Jul 13. Curr Opin Struct Biol. 2024. PMID: 39003916 Review.
-
Synthesis and Characterization of Click Chemical Probes for Single-Cell Resolution Detection of Epichaperomes in Neurodegenerative Disorders.Biomedicines. 2024 Jun 4;12(6):1252. doi: 10.3390/biomedicines12061252. Biomedicines. 2024. PMID: 38927459 Free PMC article.
-
Unraveling the Mechanism of Epichaperome Modulation by Zelavespib: Biochemical Insights on Target Occupancy and Extended Residence Time at the Site of Action.Biomedicines. 2023 Sep 22;11(10):2599. doi: 10.3390/biomedicines11102599. Biomedicines. 2023. PMID: 37892973 Free PMC article.
-
PU-H71 (NSC 750424): a molecular masterpiece that targets HSP90 in cancer and beyond.Front Pharmacol. 2024 Nov 5;15:1475998. doi: 10.3389/fphar.2024.1475998. eCollection 2024. Front Pharmacol. 2024. PMID: 39564119 Free PMC article. Review.
-
How aberrant N-glycosylation can alter protein functionality and ligand binding: An atomistic view.Structure. 2023 Aug 3;31(8):987-1004.e8. doi: 10.1016/j.str.2023.05.017. Epub 2023 Jun 20. Structure. 2023. PMID: 37343552 Free PMC article.
References
-
- Alaalm L, Crunden JL, Butcher M, Obst U, Whealy R, Williamson CE, O’Brien HE, Schaffitzel C, Ramage G, Spencer J, & Diezmann S (2021). Identification and phenotypic characterization of Hsp90 phosphorylation sites that modulate virulence traits in the major human fungal pathogen Candida albicans. Frontiers in Cellular and Infection Microbiology, 10.3389/fcimb.2021.637836. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

