Early life feeding accelerates gut microbiome maturation and suppresses acute post-weaning stress in piglets
- PMID: 34655283
- PMCID: PMC9291500
- DOI: 10.1111/1462-2920.15791
Early life feeding accelerates gut microbiome maturation and suppresses acute post-weaning stress in piglets
Abstract
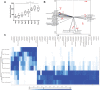
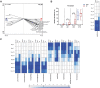
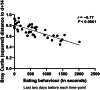
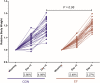
Early life microbiome perturbations can have important effects on host development, physiology and behaviour. In this longitudinal study, we evaluated the impact of early feeding on gut microbiome colonization in neonatal piglets. Early-fed (EF) piglets had access to a customized fibrous diet from 2 days after birth until weaning in addition to mother's milk, whereas control piglets suckled mother's milk only. Rectal swabs were collected at multiple time points until 6 weeks of age to investigate microbiota development using 16S rRNA gene profiling. The dynamic pre-weaning microbiota colonization was followed by a relatively stable post-weaning microbiota, represented by Prevotella, Roseburia, Faecalibacterium, Ruminococcus, Megasphaera, Catenibacterium and Subdoligranulum. EF piglets showed an accelerated microbiota maturation, characterized by increased microbial diversity, pre-weaning emergence of post-weaning-associated microbes and a more rapid decline of typical pre-weaning microbes. Furthermore, the individual eating behaviour scores of piglets quantitatively correlated with their accelerated microbiome. Importantly, EF piglets displayed a smoother relative weight gain and tended to reach a higher relative weight gain, in addition to reduced diarrhoea scores in the first week post-weaning. Overall, these findings demonstrate the beneficial impact of early feeding on microbiota development as well as pig health and performance during the weaning transition.
© 2021 The Authors. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures

Similar articles
-
Early feeding leads to molecular maturation of the gut mucosal immune system in suckling piglets.Front Immunol. 2023 May 25;14:1208891. doi: 10.3389/fimmu.2023.1208891. eCollection 2023. Front Immunol. 2023. PMID: 37304274 Free PMC article.
-
Longitudinal development of the gut microbiota in healthy and diarrheic piglets induced by age-related dietary changes.Microbiologyopen. 2019 Dec;8(12):e923. doi: 10.1002/mbo3.923. Epub 2019 Sep 9. Microbiologyopen. 2019. PMID: 31496126 Free PMC article.
-
An insight into the commercial piglet's microbial gut colonization: from birth towards weaning.Anim Microbiome. 2022 Dec 26;4(1):68. doi: 10.1186/s42523-022-00221-9. Anim Microbiome. 2022. PMID: 36572944 Free PMC article.
-
Fine-tuning of post-weaning pig microbiome structure and functionality by in-feed zinc oxide and antibiotics use.Front Cell Infect Microbiol. 2024 Feb 7;14:1354449. doi: 10.3389/fcimb.2024.1354449. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38384302 Free PMC article. Review.
-
Piglet gut microbial shifts early in life: causes and effects.J Anim Sci Biotechnol. 2019 Jan 14;10:1. doi: 10.1186/s40104-018-0308-3. eCollection 2019. J Anim Sci Biotechnol. 2019. PMID: 30651985 Free PMC article. Review.
Cited by
-
Review on Preventive Measures to Reduce Post-Weaning Diarrhoea in Piglets.Animals (Basel). 2022 Sep 27;12(19):2585. doi: 10.3390/ani12192585. Animals (Basel). 2022. PMID: 36230326 Free PMC article. Review.
-
Delayed Microbial Maturation Durably Exacerbates Th17-driven Asthma in Mice.Am J Respir Cell Mol Biol. 2023 May;68(5):498-510. doi: 10.1165/rcmb.2022-0367OC. Am J Respir Cell Mol Biol. 2023. PMID: 36622830 Free PMC article.
-
Impact of Early Weaning on Development of the Swine Gut Microbiome.Microorganisms. 2023 Jul 5;11(7):1753. doi: 10.3390/microorganisms11071753. Microorganisms. 2023. PMID: 37512925 Free PMC article. Review.
-
The Early Life Microbiota Is Not a Major Factor Underlying the Susceptibility to Postweaning Diarrhea in Piglets.Microbiol Spectr. 2023 Aug 17;11(4):e0069423. doi: 10.1128/spectrum.00694-23. Epub 2023 Jun 26. Microbiol Spectr. 2023. PMID: 37358441 Free PMC article.
-
Composition, function, and timing: exploring the early-life gut microbiota in piglets for probiotic interventions.J Anim Sci Biotechnol. 2023 Nov 13;14(1):143. doi: 10.1186/s40104-023-00943-z. J Anim Sci Biotechnol. 2023. PMID: 37957747 Free PMC article.
References
-
- Adeleye, O.O. , Guy, J.H. , and Edwards, S.A. (2014) Exploratory behaviour and performance of piglets fed novel flavoured creep in two housing systems. Anim Feed Sci Technol 191: 91–97.
-
- Alain, B.P.E. , Chae, J.P. , Balolong, M.P. , Bum Kim, H. , and Kang, D.K. (2014) Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J Gen Appl Microbiol 60: 140–146. - PubMed
-
- Bruininx, E.M.A.M. , Binnendijk, G.P. , Schrama, J.W. , Den Hartog, L.A. , Everts, H. , and Beynen, A.C. (2002) Effect of creep feed consumption on individual feed intake characteristics and performance of group‐housed weanling pigs. J Anim Sci 80: 1413–1418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

