TNF-α-activated eNOS signaling increases leukocyte adhesion through the S-nitrosylation pathway
- PMID: 34652985
- PMCID: PMC8782658
- DOI: 10.1152/ajpheart.00065.2021
TNF-α-activated eNOS signaling increases leukocyte adhesion through the S-nitrosylation pathway
Abstract
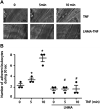
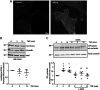
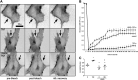
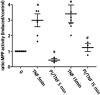
Nitric oxide (NO) is a key factor in inflammation. Endothelial nitric oxide synthase (eNOS), whose activity increases after stimulation with proinflammatory cytokines, produces NO in endothelium. NO activates two pathways: 1) soluble guanylate cyclase-protein kinase G and 2) S-nitrosylation (NO-induced modification of free-thiol cysteines in proteins). S-nitrosylation affects phosphorylation, localization, and protein interactions. NO is classically described as a negative regulator of leukocyte adhesion to endothelial cells. However, agonists activating NO production induce a fast leukocyte adhesion, which suggests that NO might positively regulate leukocyte adhesion. We tested the hypothesis that eNOS-induced NO promotes leukocyte adhesion through the S-nitrosylation pathway. We stimulated leukocyte adhesion to endothelium in vitro and in vivo using tumor necrosis factor-α (TNF-α) as proinflammatory agonist. ICAM-1 changes were evaluated by immunofluorescence, subcellular fractionation, immunoprecipitation, and fluorescence recovery after photobleaching (FRAP). Protein kinase Cζ (PKCζ) activity and S-nitrosylation were evaluated by Western blot analysis and biotin switch method, respectively. TNF-α, at short times of stimulation, activated the eNOS S-nitrosylation pathway and caused leukocyte adhesion to endothelial cells in vivo and in vitro. TNF-α-induced NO led to changes in ICAM-1 at the cell surface, which are characteristic of clustering. TNF-α-induced NO also produced S-nitrosylation and phosphorylation of PKCζ, association of PKCζ with ICAM-1, and ICAM-1 phosphorylation. The inhibition of PKCζ blocked leukocyte adhesion induced by TNF-α. Mass spectrometry analysis of purified PKCζ identified cysteine 503 as the only S-nitrosylated residue in the kinase domain of the protein. Our results reveal a new eNOS S-nitrosylation-dependent mechanism that induces leukocyte adhesion and suggests that S-nitrosylation of PKCζ may be an important regulatory step in early leukocyte adhesion in inflammation.NEW & NOTEWORTHY Contrary to the well-established inhibitory role of NO in leukocyte adhesion, we demonstrate a positive role of nitric oxide in this process. We demonstrate that NO induced by eNOS after TNF-α treatment induces early leukocyte adhesion activating the S-nitrosylation pathway. Our data suggest that PKCζ S-nitrosylation may be a key step in this process.
Keywords: S-nitrosylation; leukocyte adhesion; nitric oxide; protein kinase.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1.Circ Res. 2003 May 30;92(10):1089-97. doi: 10.1161/01.RES.0000072971.88704.CB. Epub 2003 Apr 24. Circ Res. 2003. PMID: 12714560
-
S-Nitrosylation in endothelial cells contributes to tumor cell adhesion and extravasation during breast cancer metastasis.Biol Res. 2023 Sep 29;56(1):51. doi: 10.1186/s40659-023-00461-2. Biol Res. 2023. PMID: 37773178 Free PMC article.
-
Impressic Acid, a Lupane-Type Triterpenoid from Acanthopanax koreanum, Attenuates TNF-α-Induced Endothelial Dysfunction via Activation of eNOS/NO Pathway.Int J Mol Sci. 2019 Nov 16;20(22):5772. doi: 10.3390/ijms20225772. Int J Mol Sci. 2019. PMID: 31744135 Free PMC article.
-
Proteomic identification of S-nitrosylated Golgi proteins: new insights into endothelial cell regulation by eNOS-derived NO.PLoS One. 2012;7(2):e31564. doi: 10.1371/journal.pone.0031564. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363674 Free PMC article.
-
Endothelial Nitric Oxide Synthase-Derived Nitric Oxide Prevents Dihydrofolate Reductase Degradation via Promoting S-Nitrosylation.Arterioscler Thromb Vasc Biol. 2015 Nov;35(11):2366-73. doi: 10.1161/ATVBAHA.115.305796. Epub 2015 Sep 17. Arterioscler Thromb Vasc Biol. 2015. PMID: 26381869 Free PMC article.
Cited by
-
Unveiling the Mechanisms of Bone Marrow Toxicity Induced by Lead Acetate Exposure.Biol Trace Elem Res. 2024 Mar;202(3):1041-1066. doi: 10.1007/s12011-023-03733-w. Epub 2023 Jun 28. Biol Trace Elem Res. 2024. PMID: 37378799
-
The Role of Oxidants in Percutaneous Coronary Intervention-Induced Endothelial Dysfunction: Can We Harness Redox Signaling to Improve Clinical Outcomes?Antioxid Redox Signal. 2023 May;38(13-15):1022-1040. doi: 10.1089/ars.2022.0204. Epub 2023 Mar 7. Antioxid Redox Signal. 2023. PMID: 36641638 Free PMC article. Review.
-
Targeted microbubbles combined with low-power focused ultrasound promote the thrombolysis of acute deep vein thrombosis.Front Bioeng Biotechnol. 2023 Mar 16;11:1163405. doi: 10.3389/fbioe.2023.1163405. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37008026 Free PMC article.
-
3β-Hydroxy-5β-hydroxy-B-norcholestane-6β-carboxaldehyde (SEC-B) Induces Proinflammatory Activation of Human Endothelial Cells Associated with Nitric Oxide Production and Endothelial Nitric Oxide Synthase/Caveolin-1 Dysregulation.Antioxidants (Basel). 2022 Jun 10;11(6):1148. doi: 10.3390/antiox11061148. Antioxidants (Basel). 2022. PMID: 35740044 Free PMC article.
-
An ERK5-NRF2 Axis Mediates Senescence-Associated Stemness and Atherosclerosis.Circ Res. 2023 Jun 23;133(1):25-44. doi: 10.1161/CIRCRESAHA.122.322017. Epub 2023 Jun 2. Circ Res. 2023. PMID: 37264926 Free PMC article.
References
-
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

