Immunogenicity Evaluation of N-Glycans Recognized by HIV Broadly Neutralizing Antibodies
- PMID: 34649433
- PMCID: PMC8526942
- DOI: 10.1021/acschembio.1c00375
Immunogenicity Evaluation of N-Glycans Recognized by HIV Broadly Neutralizing Antibodies
Abstract
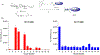
While the improved treatment of human immunodeficiency virus type 1 (HIV-1) infection is available, the development of an effective and safe prophylactic vaccine against HIV-1 is still an unrealized goal. Encouragingly, the discovery of broadly neutralizing antibodies (bNAbs) from HIV-1 positive patients that are capable of neutralizing a broad spectrum of HIV-1 isolates of various clades has accelerated the progress of vaccine development in the past few years. Some of these bNAbs recognize the N-glycans on the viral surface gp120 glycoprotein. We have been interested in using the glycan epitopes recognized by bNAbs for the development of vaccines to elicit bNAb-like antibodies with broadly neutralizing activities. Toward this goal, we have identified novel hybrid-type structures with subnanomolar avidity toward several bNAbs including PG16, PGT121, PGT128-3C, 2G12, VRC13, VRC-PG05, VRC26.25, VRC26.09, PGDM1400, 35O22, and 10-1074. Here, we report the immunogenicity evaluation of a novel hybrid glycan conjugated to carrier DTCRM197, a nontoxic mutant of the diphtheria toxin, for immunization in mice. Our results indicated that the IgG response was mainly against the chitobiose motif with nonspecific binding to a panel of N-glycans with reducing end GlcNAc-GlcNAc (chitobiose) printed on the glass slides. However, the IgM response was mainly toward the reducing end GlcNAc moiety. We further used the glycoconjugates of Man3GlcNAc2, Man5GlcNAc2, and Man9GlcNAc2 glycans for immunization, and a similar specificity pattern was observed. These findings suggest that the immunogenicity of chitobiose may interfere with the outcome of N-glycan-based vaccines, and modification may be necessary to increase the immunogenicity of the entire N-glycan epitope.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
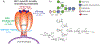
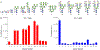
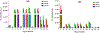
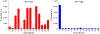
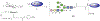


Similar articles
-
Unprecedented Role of Hybrid N-Glycans as Ligands for HIV-1 Broadly Neutralizing Antibodies.J Am Chem Soc. 2018 Apr 18;140(15):5202-5210. doi: 10.1021/jacs.8b00896. Epub 2018 Apr 9. J Am Chem Soc. 2018. PMID: 29578688
-
Broadly neutralizing antibody epitopes on HIV-1 particles are exposed after virus interaction with host cells.J Virol. 2023 Sep 28;97(9):e0071023. doi: 10.1128/jvi.00710-23. Epub 2023 Sep 8. J Virol. 2023. PMID: 37681958 Free PMC article.
-
A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120.J Virol. 2008 Jul;82(13):6359-68. doi: 10.1128/JVI.00293-08. Epub 2008 Apr 23. J Virol. 2008. PMID: 18434393 Free PMC article.
-
Strategies for eliciting multiple lineages of broadly neutralizing antibodies to HIV by vaccination.Curr Opin Virol. 2021 Dec;51:172-178. doi: 10.1016/j.coviro.2021.09.015. Epub 2021 Nov 4. Curr Opin Virol. 2021. PMID: 34742037 Free PMC article. Review.
-
Broadly Neutralizing Antibody-Guided Carbohydrate-Based HIV Vaccine Design: Challenges and Opportunities.ChemMedChem. 2016 Feb 17;11(4):357-62. doi: 10.1002/cmdc.201500498. Epub 2016 Jan 13. ChemMedChem. 2016. PMID: 26762799 Review.
Cited by
-
Synthesis and Immunological Study of N-Glycan-Bacteriophage Qβ Conjugates Reveal Dominant Antibody Responses to the Conserved Chitobiose Core.Bioconjug Chem. 2022 Jul 20;33(7):1350-1362. doi: 10.1021/acs.bioconjchem.2c00211. Epub 2022 Jun 10. Bioconjug Chem. 2022. PMID: 35687881 Free PMC article.
-
Synthetic Neoglycoconjugates of Hepta- and Nonamannoside Ligands for Eliciting Oligomannose-Specific HIV-1-Neutralizing Antibodies.Chembiochem. 2022 Apr 5;23(7):e202200061. doi: 10.1002/cbic.202200061. Epub 2022 Feb 11. Chembiochem. 2022. PMID: 35104013 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

