Perturbing HIV-1 Ribosomal Frameshifting Frequency Reveals a cis Preference for Gag-Pol Incorporation into Assembling Virions
- PMID: 34643428
- PMCID: PMC8754204
- DOI: 10.1128/JVI.01349-21
Perturbing HIV-1 Ribosomal Frameshifting Frequency Reveals a cis Preference for Gag-Pol Incorporation into Assembling Virions
Abstract
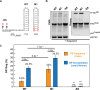
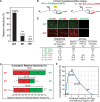
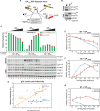
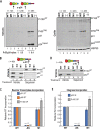
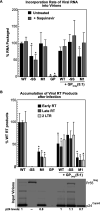
HIV-1 virion production is driven by Gag and Gag-Pol (GP) proteins, with Gag forming the bulk of the capsid and driving budding, while GP binds Gag to deliver the essential virion enzymes protease, reverse transcriptase, and integrase. Virion GP levels are traditionally thought to reflect the relative abundances of GP and Gag in cells (∼1:20), dictated by the frequency of a -1 programmed ribosomal frameshifting (PRF) event occurring in gag-pol mRNAs. Here, we exploited a panel of PRF mutant viruses to show that mechanisms in addition to PRF regulate GP incorporation into virions. First, we show that GP is enriched ∼3-fold in virions relative to cells, with viral infectivity being better maintained at subphysiological levels of GP than when GP levels are too high. Second, we report that GP is more efficiently incorporated into virions when Gag and GP are synthesized in cis (i.e., from the same gag-pol mRNA) than in trans, suggesting that Gag/GP translation and assembly are spatially coupled processes. Third, we show that, surprisingly, virions exhibit a strong upper limit to trans-delivered GP incorporation; an adaptation that appears to allow the virus to temper defects to GP/Gag cleavage that may negatively impact reverse transcription. Taking these results together, we propose a "weighted Goldilocks" scenario for HIV-1 GP incorporation, wherein combined mechanisms of GP enrichment and exclusion buffer virion infectivity over a broad range of local GP concentrations. These results provide new insights into the HIV-1 virion assembly pathway relevant to the anticipated efficacy of PRF-targeted antiviral strategies. IMPORTANCE HIV-1 infectivity requires incorporation of the Gag-Pol (GP) precursor polyprotein into virions during the process of virus particle assembly. Mechanisms dictating GP incorporation into assembling virions are poorly defined, with GP levels in virions traditionally thought to solely reflect relative levels of Gag and GP expressed in cells, dictated by the frequency of a -1 programmed ribosomal frameshifting (PRF) event that occurs in gag-pol mRNAs. Herein, we provide experimental support for a "weighted Goldilocks" scenario for GP incorporation, wherein the virus exploits both random and nonrandom mechanisms to buffer infectivity over a wide range of GP expression levels. These mechanistic data are relevant to ongoing efforts to develop antiviral strategies targeting PRF frequency and/or HIV-1 virion maturation.
Keywords: Gag; Gag-Pol; HIV; PRF; cis-acting RNA element; protease; reverse transcription; ribosomal frameshift; virion; virus assembly.
Figures

Similar articles
-
Stability of HIV Frameshift Site RNA Correlates with Frameshift Efficiency and Decreased Virus Infectivity.J Virol. 2016 Jul 11;90(15):6906-6917. doi: 10.1128/JVI.00149-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27194769 Free PMC article.
-
HIV-1 Mutant Assembly, Processing and Infectivity Expresses Pol Independent of Gag.Viruses. 2020 Jan 2;12(1):54. doi: 10.3390/v12010054. Viruses. 2020. PMID: 31906562 Free PMC article.
-
Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly.J Virol. 2017 Feb 28;91(6):e02315-16. doi: 10.1128/JVI.02315-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053097 Free PMC article.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
-
A review on architecture of the gag-pol ribosomal frameshifting RNA in human immunodeficiency virus: a variability survey of virus genotypes.J Biomol Struct Dyn. 2017 Jun;35(8):1629-1653. doi: 10.1080/07391102.2016.1194231. Epub 2016 Aug 2. J Biomol Struct Dyn. 2017. PMID: 27485859 Review.
Cited by
-
Shiftless, a Critical Piece of the Innate Immune Response to Viral Infection.Viruses. 2022 Jun 20;14(6):1338. doi: 10.3390/v14061338. Viruses. 2022. PMID: 35746809 Free PMC article. Review.
-
Advances in HIV-1 Assembly.Viruses. 2022 Feb 26;14(3):478. doi: 10.3390/v14030478. Viruses. 2022. PMID: 35336885 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

