Enhanced Oxytetracycline Production by Streptomyces rimosus in Submerged Co-Cultures with Streptomyces noursei
- PMID: 34641580
- PMCID: PMC8512450
- DOI: 10.3390/molecules26196036
Enhanced Oxytetracycline Production by Streptomyces rimosus in Submerged Co-Cultures with Streptomyces noursei
Abstract
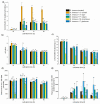
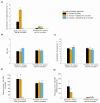
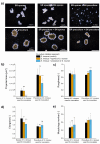
In the present study, Streptomyces rimosus was confronted with Streptomyces noursei, Penicillium rubens, Aspergillus niger, Chaetomium globosum, or Mucor racemosus in two-species submerged co-cultures in shake flasks with the goal of evaluating the oxytetracycline production and morphological development. The co-culture of S. rimosus with S. noursei exhibited stimulation in oxytetracycline biosynthesis compared with the S. rimosus monoculture, whereas the presence of M. racemosus resulted in a delay in antibiotic production. Different strategies of initiating the "S. rimosus + S. noursei" co-cultures were tested. The improvement in terms of oxytetracycline titers was recorded in the cases where S. noursei was co-inoculated with S. rimosus in the form of spores. As the observed morphological changes were not unique to the co-culture involving S. noursei, there was no evidence that the improvement of oxytetracycline levels could be attributed mainly to morphology-related characteristics.
Keywords: Streptomyces noursei; Streptomyces rimosus; co-culture; oxytetracycline; rimocidin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effects of the Coculture Initiation Method on the Production of Secondary Metabolites in Bioreactor Cocultures of Penicillium rubens and Streptomyces rimosus.Molecules. 2023 Aug 13;28(16):6044. doi: 10.3390/molecules28166044. Molecules. 2023. PMID: 37630296 Free PMC article.
-
Oxytetracycline hyper-production through targeted genome reduction of Streptomyces rimosus.mSystems. 2024 May 16;9(5):e0025024. doi: 10.1128/msystems.00250-24. Epub 2024 Apr 2. mSystems. 2024. PMID: 38564716 Free PMC article.
-
Phosphoproteome Dynamics of Streptomyces rimosus during Submerged Growth and Antibiotic Production.mSystems. 2022 Oct 26;7(5):e0019922. doi: 10.1128/msystems.00199-22. Epub 2022 Sep 12. mSystems. 2022. PMID: 36094082 Free PMC article.
-
Genetics of Streptomyces rimosus, the oxytetracycline producer.Microbiol Mol Biol Rev. 2006 Sep;70(3):704-28. doi: 10.1128/MMBR.00004-06. Microbiol Mol Biol Rev. 2006. PMID: 16959966 Free PMC article. Review.
-
A bioprocess perspective on the production of secondary metabolites by Streptomyces in submerged co-cultures.World J Microbiol Biotechnol. 2021 Sep 7;37(10):171. doi: 10.1007/s11274-021-03141-z. World J Microbiol Biotechnol. 2021. PMID: 34490503 Free PMC article. Review.
Cited by
-
Computation-aided studies related to the induction of specialized metabolite biosynthesis in microbial co-cultures: An introductory overview.Comput Struct Biotechnol J. 2023 Aug 16;21:4021-4029. doi: 10.1016/j.csbj.2023.08.011. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37649711 Free PMC article. Review.
-
Effects of the Coculture Initiation Method on the Production of Secondary Metabolites in Bioreactor Cocultures of Penicillium rubens and Streptomyces rimosus.Molecules. 2023 Aug 13;28(16):6044. doi: 10.3390/molecules28166044. Molecules. 2023. PMID: 37630296 Free PMC article.
-
Investigating the Stirred Tank Bioreactor Co-Cultures of the Secondary Metabolite Producers Streptomyces noursei and Penicillium rubens.Biomolecules. 2023 Dec 5;13(12):1748. doi: 10.3390/biom13121748. Biomolecules. 2023. PMID: 38136619 Free PMC article.
-
Production of secondary metabolites in stirred tank bioreactor co-cultures of Streptomyces noursei and Aspergillus terreus.Front Bioeng Biotechnol. 2022 Sep 29;10:1011220. doi: 10.3389/fbioe.2022.1011220. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246390 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

