Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development
- PMID: 34641563
- PMCID: PMC8513016
- DOI: 10.3390/molecules26196019
Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development
Abstract
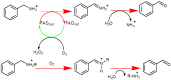
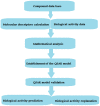
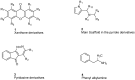
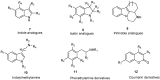
Monoamine oxidases (MAOs) are oxidative enzymes that catalyze the conversion of biogenic amines into their corresponding aldehydes and ketones through oxidative deamination. Owing to the crucial role of MAOs in maintaining functional levels of neurotransmitters, the implications of its distorted activity have been associated with numerous neurological diseases. Recently, an unanticipated role of MAOs in tumor progression and metastasis has been reported. The chemical inhibition of MAOs might be a valuable therapeutic approach for cancer treatment. In this review, we reported computational approaches exploited in the design and development of selective MAO inhibitors accompanied by their biological activities. Additionally, we generated a pharmacophore model for MAO-A active inhibitors to identify the structural motifs to invoke an activity.
Keywords: QSAR; cancer; inhibitors; metastasis; monoamine oxidase; pharmacophore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

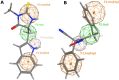
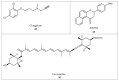
Similar articles
-
Quantitative Structure-Activity Relationship (QSAR) Studies for the Inhibition of MAOs.Comb Chem High Throughput Screen. 2020;23(9):887-897. doi: 10.2174/1386207323666200324173231. Comb Chem High Throughput Screen. 2020. PMID: 32208114 Review.
-
Monoamine Oxidase Inhibitors: From Classic to New Clinical Approaches.Handb Exp Pharmacol. 2021;264:229-259. doi: 10.1007/164_2020_384. Handb Exp Pharmacol. 2021. PMID: 32852645 Review.
-
A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer's disease agents: A review.Eur J Med Chem. 2020 Nov 15;206:112787. doi: 10.1016/j.ejmech.2020.112787. Epub 2020 Sep 1. Eur J Med Chem. 2020. PMID: 32942081 Review.
-
A second life for MAO inhibitors? From CNS diseases to anticancer therapy.Eur J Med Chem. 2024 Mar 5;267:116180. doi: 10.1016/j.ejmech.2024.116180. Epub 2024 Jan 24. Eur J Med Chem. 2024. PMID: 38290352 Review.
-
Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease.Curr Opin Pharmacol. 2017 Apr;33:64-69. doi: 10.1016/j.coph.2017.04.003. Epub 2017 May 18. Curr Opin Pharmacol. 2017. PMID: 28528298 Review.
Cited by
-
Molecular Investigation of the Antitumor Effects of Monoamine Oxidase Inhibitors in Breast Cancer Cells.Biomed Res Int. 2023 Oct 5;2023:2592691. doi: 10.1155/2023/2592691. eCollection 2023. Biomed Res Int. 2023. PMID: 37841082 Free PMC article.
-
Potential molecular metabolic mechanisms underlying the effects of cimifugin in gastric cancer through single-cell and bulk RNA sequencing combined with network pharmacology.J Gastrointest Oncol. 2024 Aug 31;15(4):1409-1430. doi: 10.21037/jgo-24-413. Epub 2024 Aug 19. J Gastrointest Oncol. 2024. PMID: 39279957 Free PMC article.
-
Pyrazoline Derivatives as Promising MAO-A Targeting Antidepressants: An Update.Curr Top Med Chem. 2024;24(5):401-415. doi: 10.2174/0115680266280249240126052505. Curr Top Med Chem. 2024. PMID: 38318823 Review.
-
Design, Synthesis, and Biological Evaluation of Novel MAO-A Inhibitors Targeting Lung Cancer.Molecules. 2022 Apr 30;27(9):2887. doi: 10.3390/molecules27092887. Molecules. 2022. PMID: 35566238 Free PMC article.
-
N-Acetylcysteine Attenuates Cisplatin Toxicity in the Cerebrum and Lung of Young Rats with Artificially Induced Protein Deficiency.Int J Mol Sci. 2024 Jun 5;25(11):6239. doi: 10.3390/ijms25116239. Int J Mol Sci. 2024. PMID: 38892427 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

