Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells
- PMID: 34639228
- PMCID: PMC8509224
- DOI: 10.3390/ijms221910890
Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells
Abstract
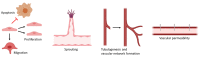
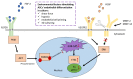
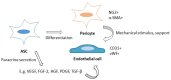
Adipose-derived mesenchymal stem/stromal cells (ASCs) are an adult stem cell population able to self-renew and differentiate into numerous cell lineages. ASCs provide a promising future for therapeutic angiogenesis due to their ability to promote blood vessel formation. Specifically, their ability to differentiate into endothelial cells (ECs) and pericyte-like cells and to secrete angiogenesis-promoting growth factors and extracellular vesicles (EVs) makes them an ideal option in cell therapy and in regenerative medicine in conditions including tissue ischemia. In recent angiogenesis research, ASCs have often been co-cultured with an endothelial cell (EC) type in order to form mature vessel-like networks in specific culture conditions. In this review, we introduce co-culture systems and co-transplantation studies between ASCs and ECs. In co-cultures, the cells communicate via direct cell-cell contact or via paracrine signaling. Most often, ASCs are found in the perivascular niche lining the vessels, where they stabilize the vascular structures and express common pericyte surface proteins. In co-cultures, ASCs modulate endothelial cells and induce angiogenesis by promoting tube formation, partly via secretion of EVs. In vivo co-transplantation of ASCs and ECs showed improved formation of functional vessels over a single cell type transplantation. Adipose tissue as a cell source for both mesenchymal stem cells and ECs for co-transplantation serves as a prominent option for therapeutic angiogenesis and blood perfusion in vivo.
Keywords: adipose-derived mesenchymal stem/stromal cell; angiogenesis; cell transplantation; co-culture; co-transplantation; endothelial cell; extracellular vesicle; ischemia; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
TGFβ signalling pathway regulates angiogenesis by endothelial cells, in an adipose-derived stromal cell/endothelial cell co-culture 3D gel model.Cell Prolif. 2015 Dec;48(6):729-37. doi: 10.1111/cpr.12222. Epub 2015 Oct 21. Cell Prolif. 2015. PMID: 26487556 Free PMC article.
-
An In Vitro Co-Culture Model of Bone Marrow Mesenchymal Stromal Cells and Peripheral Blood Mononuclear Cells Promotes the Differentiation of Myeloid Angiogenic Cells and Pericyte-Like Cells.Stem Cells Dev. 2021 Mar;30(6):309-324. doi: 10.1089/scd.2019.0171. Epub 2021 Mar 1. Stem Cells Dev. 2021. PMID: 33499756
-
Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis.PLoS One. 2022 Jan 13;17(1):e0261498. doi: 10.1371/journal.pone.0261498. eCollection 2022. PLoS One. 2022. PMID: 35025920 Free PMC article.
-
The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis.Int J Mol Sci. 2020 May 27;21(11):3790. doi: 10.3390/ijms21113790. Int J Mol Sci. 2020. PMID: 32471255 Free PMC article. Review.
-
Mesenchymal stem cell-derived extracellular vesicles: a new impetus of promoting angiogenesis in tissue regeneration.Cytotherapy. 2019 May;21(5):497-508. doi: 10.1016/j.jcyt.2018.11.012. Cytotherapy. 2019. PMID: 31079806 Review.
Cited by
-
Mesenchymal Stem Cells and Secretome as a New Possible Approach to Treat Cartilage Damage: An In Vitro Study.Biomolecules. 2024 Aug 26;14(9):1068. doi: 10.3390/biom14091068. Biomolecules. 2024. PMID: 39334835 Free PMC article.
-
Regenerative Potential of Mesenchymal Stem Cells' (MSCs) Secretome for Liver Fibrosis Therapies.Int J Mol Sci. 2021 Dec 10;22(24):13292. doi: 10.3390/ijms222413292. Int J Mol Sci. 2021. PMID: 34948088 Free PMC article. Review.
-
Molecular Morphology and Function of Stromal Cells.Int J Mol Sci. 2021 Dec 14;22(24):13422. doi: 10.3390/ijms222413422. Int J Mol Sci. 2021. PMID: 34948214 Free PMC article.
-
Angiogenic Potential of Human Adipose-Derived Mesenchymal Stromal Cells in Nanofibrillated Cellulose Hydrogel.Biomedicines. 2022 Oct 15;10(10):2584. doi: 10.3390/biomedicines10102584. Biomedicines. 2022. PMID: 36289846 Free PMC article.
-
Comparison of the Behavior of Perivascular Cells (Pericytes and CD34+ Stromal Cell/Telocytes) in Sprouting and Intussusceptive Angiogenesis.Int J Mol Sci. 2022 Aug 12;23(16):9010. doi: 10.3390/ijms23169010. Int J Mol Sci. 2022. PMID: 36012273 Free PMC article. Review.
References
-
- Traktuev D.O., Merfeld-Clauss S., Li J., Kolonin M., Arap W., Pasqualini R., Johnstone B.H., March K.L. A Population of Multipotent CD34-Positive Adipose Stromal Cells Share Pericyte and Mesenchymal Surface Markers, Reside in a Periendothelial Location, and Stabilize Endothelial Networks. Circ. Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

