Molecular Imaging of Human Skeletal Myoblasts (huSKM) in Mouse Post-Infarction Myocardium
- PMID: 34639225
- PMCID: PMC8509689
- DOI: 10.3390/ijms221910885
Molecular Imaging of Human Skeletal Myoblasts (huSKM) in Mouse Post-Infarction Myocardium
Abstract
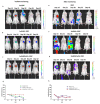
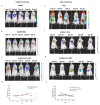
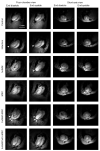
Current treatment protocols for myocardial infarction improve the outcome of disease to some extent but do not provide the clue for full regeneration of the heart tissues. An increasing body of evidence has shown that transplantation of cells may lead to some organ recovery. However, the optimal stem cell population has not been yet identified. We would like to propose a novel pro-regenerative treatment for post-infarction heart based on the combination of human skeletal myoblasts (huSkM) and mesenchymal stem cells (MSCs). huSkM native or overexpressing gene coding for Cx43 (huSKMCx43) alone or combined with MSCs were delivered in four cellular therapeutic variants into the healthy and post-infarction heart of mice while using molecular reporter probes. Single-Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) performed right after cell delivery and 24 h later revealed a trend towards an increase in the isotopic uptake in the post-infarction group of animals treated by a combination of huSkMCx43 with MSC. Bioluminescent imaging (BLI) showed the highest increase in firefly luciferase (fluc) signal intensity in post-infarction heart treated with combination of huSkM and MSCs vs. huSkM alone (p < 0.0001). In healthy myocardium, however, nanoluciferase signal (nanoluc) intensity varied markedly between animals treated with stem cell populations either alone or in combinations with the tendency to be simply decreased. Therefore, our observations seem to show that MSCs supported viability, engraftment, and even proliferation of huSkM in the post-infarction heart.
Keywords: Bioluminescent Imaging; Magnetic Resonance Imaging; Single-Photon Emission Computed Tomography/Computed Tomography; human skeletal myoblasts; mesenchymal stem cells; promoter reporter gene; technetium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Molecular imaging of myogenic stem/progenitor cells with [18F]-FHBG PET/CT system in SCID mice model of post-infarction heart.Sci Rep. 2021 Oct 6;11(1):19825. doi: 10.1038/s41598-021-98861-5. Sci Rep. 2021. PMID: 34615887 Free PMC article.
-
Mesenchymal stem cells from ischemic heart disease patients improve left ventricular function after acute myocardial infarction.Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2438-47. doi: 10.1152/ajpheart.00365.2007. Epub 2007 Jul 20. Am J Physiol Heart Circ Physiol. 2007. PMID: 17644573
-
Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model.Tissue Eng Part A. 2014 Feb;20(3-4):728-39. doi: 10.1089/ten.TEA.2012.0534. Epub 2014 Jan 3. Tissue Eng Part A. 2014. PMID: 24164292 Free PMC article.
-
Molecular imaging of cardiac remodelling after myocardial infarction.Basic Res Cardiol. 2018 Jan 17;113(2):10. doi: 10.1007/s00395-018-0668-z. Basic Res Cardiol. 2018. PMID: 29344827 Free PMC article. Review.
-
Molecular imaging of myocardial infarction.Basic Res Cardiol. 2014 Jan;109(1):397. doi: 10.1007/s00395-013-0397-2. Epub 2013 Dec 10. Basic Res Cardiol. 2014. PMID: 24322905 Review.
Cited by
-
Optimization of human myoblasts culture under different media conditions for application in the in vitro studies.Am J Stem Cells. 2022 Feb 15;11(1):1-11. eCollection 2022. Am J Stem Cells. 2022. PMID: 35295592 Free PMC article.
References
-
- Guan X., Xu W., Zhang H., Wang Q., Yu J., Zhang R., Chen Y., Xia Y., Wang J., Wang D. Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Res. Ther. 2020;11:73. doi: 10.1186/s13287-020-01602-0. - DOI - PMC - PubMed
-
- He K.L., Yi G.H., Sherman W., Zhou H., Zhang G.P., Gu A., Kao R., Haimes H.B., Harvey J., Roos E., et al. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J. Hear Lung Transplant. 2005;24:1940–1949. doi: 10.1016/j.healun.2005.02.024. - DOI - PubMed
-
- Gavira J.J., Nasarre E., Abizanda G., Pérez-Ilzarbe M., de Martino-Rodriguez A., de Jalón J.A.G., Mazo M., Macias A., García-Bolao I., Pelacho B., et al. Repeated implantation of skeletal myoblast in a swine model of chronic myocardial infarion. Eur. Heart J. 2010;31:1013–1021. doi: 10.1093/eurheartj/ehp342. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

