Deletion of Neuronal CuZnSOD Accelerates Age-Associated Muscle Mitochondria and Calcium Handling Dysfunction That Is Independent of Denervation and Precedes Sarcopenia
- PMID: 34639076
- PMCID: PMC8509582
- DOI: 10.3390/ijms221910735
Deletion of Neuronal CuZnSOD Accelerates Age-Associated Muscle Mitochondria and Calcium Handling Dysfunction That Is Independent of Denervation and Precedes Sarcopenia
Abstract
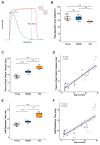
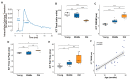
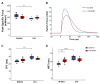
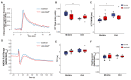
Skeletal muscle suffers atrophy and weakness with aging. Denervation, oxidative stress, and mitochondrial dysfunction are all proposed as contributors to age-associated muscle loss, but connections between these factors have not been established. We examined contractility, mitochondrial function, and intracellular calcium transients (ICTs) in muscles of mice throughout the life span to define their sequential relationships. We performed these same measures and analyzed neuromuscular junction (NMJ) morphology in mice with postnatal deletion of neuronal Sod1 (i-mn-Sod1-/- mice), previously shown to display accelerated age-associated muscle loss and exacerbation of denervation in old age, to test relationships between neuronal redox homeostasis, NMJ degeneration and mitochondrial function. In control mice, the amount and rate of the decrease in mitochondrial NADH during contraction was greater in middle than young age although force was not reduced, suggesting decreased efficiency of NADH utilization prior to the onset of weakness. Declines in both the peak of the ICT and force were observed in old age. Muscles of i-mn-Sod1-/- mice showed degeneration of mitochondrial and calcium handling functions in middle-age and a decline in force generation to a level not different from the old control mice, with maintenance of NMJ morphology. Together, the findings support the conclusion that muscle mitochondrial function decreases during aging and in response to altered neuronal redox status prior to NMJ deterioration or loss of mass and force suggesting mitochondrial defects contribute to sarcopenia independent of denervation.
Keywords: NADH; calcium; denervation; oxidative stress; sarcopenia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Transgenic expression of SOD1 specifically in neurons of Sod1 deficient mice prevents defects in muscle mitochondrial function and calcium handling.Free Radic Biol Med. 2021 Mar;165:299-311. doi: 10.1016/j.freeradbiomed.2021.01.047. Epub 2021 Feb 6. Free Radic Biol Med. 2021. PMID: 33561489 Free PMC article.
-
Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration.FASEB J. 2010 May;24(5):1376-90. doi: 10.1096/fj.09-146308. Epub 2009 Dec 29. FASEB J. 2010. PMID: 20040516 Free PMC article.
-
Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia.J Cachexia Sarcopenia Muscle. 2021 Dec;12(6):1582-1596. doi: 10.1002/jcsm.12768. Epub 2021 Sep 24. J Cachexia Sarcopenia Muscle. 2021. PMID: 34559475 Free PMC article.
-
Accelerated sarcopenia in Cu/Zn superoxide dismutase knockout mice.Free Radic Biol Med. 2019 Feb 20;132:19-23. doi: 10.1016/j.freeradbiomed.2018.06.032. Epub 2018 Jul 2. Free Radic Biol Med. 2019. PMID: 30670156 Free PMC article. Review.
-
Age-associated alterations of the neuromuscular junction.Exp Gerontol. 2011 Feb-Mar;46(2-3):193-8. doi: 10.1016/j.exger.2010.08.029. Epub 2010 Sep 18. Exp Gerontol. 2011. PMID: 20854887 Free PMC article. Review.
Cited by
-
GCPII Inhibition Promotes Remyelination after Peripheral Nerve Injury in Aged Mice.Int J Mol Sci. 2024 Jun 23;25(13):6893. doi: 10.3390/ijms25136893. Int J Mol Sci. 2024. PMID: 39000003 Free PMC article.
-
Identification of the cuproptosis-related hub genes and therapeutic agents for sarcopenia.Front Genet. 2023 Mar 17;14:1136763. doi: 10.3389/fgene.2023.1136763. eCollection 2023. Front Genet. 2023. PMID: 37007946 Free PMC article.
-
Unraveling the causes of sarcopenia: Roles of neuromuscular junction impairment and mitochondrial dysfunction.Physiol Rep. 2024 Jan;12(1):e15917. doi: 10.14814/phy2.15917. Physiol Rep. 2024. PMID: 38225199 Free PMC article. Review.
-
CUL3 and COPS5 Related to the Ubiquitin-Proteasome Pathway Are Potential Genes for Muscle Atrophy in Mice.Evid Based Complement Alternat Med. 2022 Jun 30;2022:1488905. doi: 10.1155/2022/1488905. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35815279 Free PMC article.
-
Intracellular iron accumulation facilitates mycobacterial infection in old mouse macrophages.Geroscience. 2024 Apr;46(2):2739-2754. doi: 10.1007/s11357-023-01048-1. Epub 2023 Dec 30. Geroscience. 2024. PMID: 38159133 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

