The Keratinocyte as a Crucial Cell in the Predisposition, Onset, Progression, Therapy and Study of the Atopic Dermatitis
- PMID: 34639001
- PMCID: PMC8509070
- DOI: 10.3390/ijms221910661
The Keratinocyte as a Crucial Cell in the Predisposition, Onset, Progression, Therapy and Study of the Atopic Dermatitis
Abstract
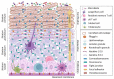
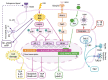
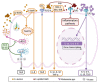
The keratinocyte (KC) is the main functional and structural component of the epidermis, the most external layer of the skin that is highly specialized in defense against external agents, prevention of leakage of body fluids and retention of internal water within the cells. Altered epidermal barrier and aberrant KC differentiation are involved in the pathophysiology of several skin diseases, such as atopic dermatitis (AD). AD is a chronic inflammatory disease characterized by cutaneous and systemic immune dysregulation and skin microbiota dysbiosis. Nevertheless, the pathological mechanisms of this complex disease remain largely unknown. In this review, we summarize current knowledge about the participation of the KC in different aspects of the AD. We provide an overview of the genetic predisposing and environmental factors, inflammatory molecules and signaling pathways of the KC that participate in the physiopathology of the AD. We also analyze the link among the KC, the microbiota and the inflammatory response underlying acute and chronic skin AD lesions.
Keywords: allergic inflammatory response; atopic dermatitis; in vitro atopic dermatitis models; keratinocyte; keratinocyte differentiation; pharmacological therapy; skin microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis.Int J Mol Sci. 2020 Apr 20;21(8):2867. doi: 10.3390/ijms21082867. Int J Mol Sci. 2020. PMID: 32326002 Free PMC article. Review.
-
The role of innate immune signaling in the pathogenesis of atopic dermatitis and consequences for treatments.Semin Immunopathol. 2016 Jan;38(1):29-43. doi: 10.1007/s00281-015-0544-y. Epub 2015 Nov 16. Semin Immunopathol. 2016. PMID: 26573298 Review.
-
[The research for atopic dermatitis: up to date].Nihon Rinsho. 2014 Aug;72(8):1503-9. Nihon Rinsho. 2014. PMID: 25167760 Review. Japanese.
-
Keratinocytes: innate immune cells in atopic dermatitis.Clin Exp Immunol. 2021 Jun;204(3):296-309. doi: 10.1111/cei.13575. Epub 2021 Feb 15. Clin Exp Immunol. 2021. PMID: 33460469 Free PMC article. Review.
-
miR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation.Allergy. 2019 Nov;74(11):2146-2156. doi: 10.1111/all.13849. Epub 2019 Jun 6. Allergy. 2019. PMID: 31049964 Free PMC article.
Cited by
-
The Efficacy of Imiquimod-Induced Psoriasis Model on Murine Cells.Cureus. 2024 Jun 22;16(6):e62914. doi: 10.7759/cureus.62914. eCollection 2024 Jun. Cureus. 2024. PMID: 39040747 Free PMC article.
-
In Vitro Anti-Inflammatory and Skin Protective Effects of Codium fragile Extract on Macrophages and Human Keratinocytes in Atopic Dermatitis.J Microbiol Biotechnol. 2024 Apr 28;34(4):940-948. doi: 10.4014/jmb.2312.12002. Epub 2024 Jan 17. J Microbiol Biotechnol. 2024. PMID: 38314445 Free PMC article.
-
Veronica persica Ethanol Extract Ameliorates Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Inflammation in Mice, Likely by Inducing Nrf2/HO-1 Signaling.Antioxidants (Basel). 2023 Jun 13;12(6):1267. doi: 10.3390/antiox12061267. Antioxidants (Basel). 2023. PMID: 37371997 Free PMC article.
-
Repressive Control of Keratinocyte Cytoplasmic Inflammatory Signaling.Int J Mol Sci. 2023 Jul 26;24(15):11943. doi: 10.3390/ijms241511943. Int J Mol Sci. 2023. PMID: 37569318 Free PMC article. Review.
-
Emerging Biologic Therapies for the Treatment of Atopic Dermatitis.Drugs. 2024 Nov;84(11):1379-1394. doi: 10.1007/s40265-024-02095-4. Epub 2024 Oct 4. Drugs. 2024. PMID: 39365406 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

