Epigenetic Alterations in Podocytes in Diabetic Nephropathy
- PMID: 34630127
- PMCID: PMC8497789
- DOI: 10.3389/fphar.2021.759299
Epigenetic Alterations in Podocytes in Diabetic Nephropathy
Abstract
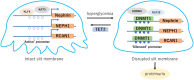
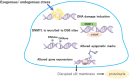
Recently, epigenetic alterations have been shown to be involved in the pathogenesis of diabetes and its complications. Kidney podocytes, which are glomerular epithelial cells, are important cells that form a slit membrane-a barrier for proteinuria. Podocytes are terminally differentiated cells without cell division or replenishment abilities. Therefore, podocyte damage is suggested to be one of the key factors determining renal prognosis. Recent studies, including ours, suggest that epigenetic changes in podocytes are associated with chronic kidney disease, including diabetic nephropathy. Furthermore, the association between DNA damage repair and epigenetic changes in diabetic podocytes has been demonstrated. Detection of podocyte DNA damage and epigenetic changes using human samples, such as kidney biopsy and urine-derived cells, may be a promising strategy for estimating kidney damage and renal prognoses in patients with diabetes. Targeting epigenetic podocyte changes and associated DNA damage may become a novel therapeutic strategy for preventing progression to end-stage renal disease (ESRD) and provide a possible prognostic marker in diabetic nephropathy. This review summarizes recent advances regarding epigenetic changes, especially DNA methylation, in podocytes in diabetic nephropathy and addresses detection of these alterations in human samples. Additionally, we focused on DNA damage, which is increased under high-glucose conditions and associated with the generation of epigenetic changes in podocytes. Furthermore, epigenetic memory in diabetes is discussed. Understanding the role of epigenetic changes in podocytes in diabetic nephropathy may be of great importance considering the increasing diabetic nephropathy patient population in an aging society.
Keywords: DNA damage repair; diabetic nephropathy; epigenetic memory; epigenetics; podocyte.
Copyright © 2021 Sugita, Hayashi, Hishikawa and Itoh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Targeting DNA Methylation in Podocytes to Overcome Chronic Kidney Disease.Keio J Med. 2023 Sep 25;72(3):67-76. doi: 10.2302/kjm.2022-0017-IR. Epub 2023 Jun 3. Keio J Med. 2023. PMID: 37271519 Review.
-
Histone modification in podocyte injury of diabetic nephropathy.J Mol Med (Berl). 2022 Oct;100(10):1373-1386. doi: 10.1007/s00109-022-02247-7. Epub 2022 Aug 30. J Mol Med (Berl). 2022. PMID: 36040515 Review.
-
Significance of podocyte DNA damage and glomerular DNA methylation in CKD patients with proteinuria.Hypertens Res. 2023 Apr;46(4):1000-1008. doi: 10.1038/s41440-023-01169-2. Epub 2023 Jan 17. Hypertens Res. 2023. PMID: 36646881
-
Emerging role of podocyte autophagy in the progression of diabetic nephropathy.Autophagy. 2015;11(12):2385-6. doi: 10.1080/15548627.2015.1115173. Autophagy. 2015. PMID: 26565953 Free PMC article.
-
Effects of High Glucose and Lipotoxicity on Diabetic Podocytes.Nutrients. 2021 Jan 15;13(1):241. doi: 10.3390/nu13010241. Nutrients. 2021. PMID: 33467659 Free PMC article. Review.
Cited by
-
Diabetic Proteinuria Revisited: Updated Physiologic Perspectives.Cells. 2022 Sep 18;11(18):2917. doi: 10.3390/cells11182917. Cells. 2022. PMID: 36139492 Free PMC article. Review.
-
A Narrative Review of New Treatment Options for Diabetic Nephropathy.Cureus. 2023 Jan 1;15(1):e33235. doi: 10.7759/cureus.33235. eCollection 2023 Jan. Cureus. 2023. PMID: 36733548 Free PMC article. Review.
-
Epigenetics in Obesity and Diabetes Mellitus: New Insights.Nutrients. 2023 Feb 4;15(4):811. doi: 10.3390/nu15040811. Nutrients. 2023. PMID: 36839169 Free PMC article. Review.
-
Protective Effect of Astragaloside IV against Cadmium-Induced Damage on Mouse Renal Podocytes (MPC5).Molecules. 2023 Jun 21;28(13):4897. doi: 10.3390/molecules28134897. Molecules. 2023. PMID: 37446560 Free PMC article.
-
Tanshinone IIA Promoted Autophagy and Inhibited Inflammation to Alleviate Podocyte Injury in Diabetic Nephropathy.Diabetes Metab Syndr Obes. 2024 Jul 22;17:2709-2724. doi: 10.2147/DMSO.S464015. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39072344 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

