Knockdown of m6A Reader IGF2BP3 Inhibited Hypoxia-Induced Cell Migration and Angiogenesis by Regulating Hypoxia Inducible Factor-1α in Stomach Cancer
- PMID: 34621671
- PMCID: PMC8490730
- DOI: 10.3389/fonc.2021.711207
Knockdown of m6A Reader IGF2BP3 Inhibited Hypoxia-Induced Cell Migration and Angiogenesis by Regulating Hypoxia Inducible Factor-1α in Stomach Cancer
Abstract
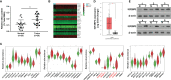
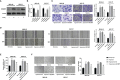
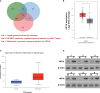
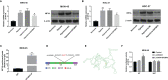
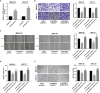
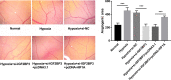
Hypoxia is a common feature of solid tumors including stomach cancer (SC) and is closely associated with cancer malignant progression. N6-methyladenosine (m6A), a common modification on RNA, is involved in the regulation of RNA fate and hypoxic responses in cancers. However, the interaction between m6A reader insulin-like growth factor-II mRNA-binding protein 3 (IGF2BP3) and SC hypoxic microenvironment is poorly defined. In the present study, expression levels of IGF2BP3 and hypoxia inducible factor-1α (HIF1A) were examined by bioinformatics analysis and RT-qPCR and western blot assays. Cell migratory ability was assessed through Transwell and wound healing assays. The angiogenic potential was evaluated by VEGF secretion, tube formation, and chick embryo chorioallantoic membrane (CAM) assays. The interaction between IGF2BP3 and HIF1A was explored using bioinformatics analysis and RIP and luciferase reporter assays. The results showed that IGF2BP3 and HIF1A were highly expressed in SC tissues and hypoxia-treated SC cells. IGF2BP3 knockdown inhibited hypoxia-induced cell migration and angiogenesis in SC. IGF2BP3 positively regulated HIF1A expression by directly binding to a specific m6A site in the coding region of HIF1A mRNA in SC cells. HIF1A overexpression abrogated the effects of IGF2BP3 knockdown on hypoxia-induced cell migration and angiogenesis in SC. In conclusion, IGF2BP3 knockdown inhibited hypoxia-induced cell migration and angiogenesis by down-regulating HIF1A in SC.
Keywords: HIF-1A; IGF2BP3; angiogenesis; hypoxia; migration; stomach cancer.
Copyright © 2021 Jiang, Li, He, Wei, Yan and Wen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer.J Exp Clin Cancer Res. 2020 Sep 29;39(1):203. doi: 10.1186/s13046-020-01714-8. J Exp Clin Cancer Res. 2020. PMID: 32993738 Free PMC article.
-
IGF2BP3/HIF1A/YAP signaling plays a role in driving acute-on-chronic liver failure through activating hepatocyte reprogramming.Cell Signal. 2023 Aug;108:110727. doi: 10.1016/j.cellsig.2023.110727. Epub 2023 May 29. Cell Signal. 2023. PMID: 37257765
-
N6-Methyladenosine Methyltransferase METTL3 Promotes Angiogenesis and Atherosclerosis by Upregulating the JAK2/STAT3 Pathway via m6A Reader IGF2BP1.Front Cell Dev Biol. 2021 Dec 7;9:731810. doi: 10.3389/fcell.2021.731810. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34950654 Free PMC article.
-
RNA N6-methyladenosine reader IGF2BP3 interacts with MYCN and facilitates neuroblastoma cell proliferation.Cell Death Discov. 2023 May 8;9(1):151. doi: 10.1038/s41420-023-01449-3. Cell Death Discov. 2023. PMID: 37156775 Free PMC article.
-
Targeting IGF2BP3 in Cancer.Int J Mol Sci. 2023 May 29;24(11):9423. doi: 10.3390/ijms24119423. Int J Mol Sci. 2023. PMID: 37298373 Free PMC article. Review.
Cited by
-
Insights into RNA N6-methyladenosine and programmed cell death in atherosclerosis.Mol Med. 2024 Sep 3;30(1):137. doi: 10.1186/s10020-024-00901-z. Mol Med. 2024. PMID: 39227813 Free PMC article. Review.
-
Identification of a Hypoxia-Angiogenesis lncRNA Signature Participating in Immunosuppression in Gastric Cancer.J Immunol Res. 2022 Aug 23;2022:5209607. doi: 10.1155/2022/5209607. eCollection 2022. J Immunol Res. 2022. PMID: 36052279 Free PMC article.
-
Role of N6-methyladenosine in tumor neovascularization.Cell Death Dis. 2024 Aug 5;15(8):563. doi: 10.1038/s41419-024-06931-z. Cell Death Dis. 2024. PMID: 39098905 Free PMC article. Review.
-
Constructing and validating of m6a-related genes prognostic signature for stomach adenocarcinoma and immune infiltration: Potential biomarkers for predicting the overall survival.Front Oncol. 2022 Dec 22;12:1050288. doi: 10.3389/fonc.2022.1050288. eCollection 2022. Front Oncol. 2022. PMID: 36620557 Free PMC article.
-
The role of Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) as m6A readers in cancer.Int J Biol Sci. 2022 Mar 28;18(7):2744-2758. doi: 10.7150/ijbs.70458. eCollection 2022. Int J Biol Sci. 2022. PMID: 35541906 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

