When Origin Matters: Properties of Mesenchymal Stromal Cells From Different Sources for Clinical Translation in Kidney Disease
- PMID: 34616756
- PMCID: PMC8488400
- DOI: 10.3389/fmed.2021.728496
When Origin Matters: Properties of Mesenchymal Stromal Cells From Different Sources for Clinical Translation in Kidney Disease
Abstract
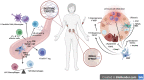
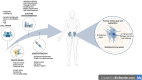
Advanced therapy medicinal products (ATMPs) offer new prospects to improve the treatment of conditions with unmet medical needs. Kidney diseases are a current major health concern with an increasing global prevalence. Chronic renal failure appears after many years of impairment, which opens a temporary window to apply novel therapeutic approaches to delay or halt disease progression. The immunomodulatory, anti-inflammatory, and pro-regenerative properties of mesenchymal stromal cells (MSCs) have sparked interest for their use in cell-based regenerative therapies. Currently, several early-phase clinical trials have been completed and many are ongoing to explore MSC safety and efficacy in a wide range of nephropathies. However, one of the current roadblocks to the clinical translation of MSC therapies relates to the lack of standardization and harmonization of MSC manufacturing protocols, which currently hinders inter-study comparability. Studies have shown that cell culture processing variables can have significant effects on MSC phenotype and functionality, and these are highly variable across laboratories. In addition, heterogeneity within MSC populations is another obstacle. Furthermore, MSCs may be isolated from several sources which adds another variable to the comparative assessment of outcomes. There is now a growing body of literature highlighting unique and distinctive properties of MSCs according to the tissue origin, and that characteristics such as donor, age, sex and underlying medical conditions may alter the therapeutic effect of MSCs. These variables must be taken into consideration when developing a cell therapy product. Having an optimal scale-up strategy for MSC manufacturing is critical for ensuring product quality while minimizing costs and time of production, as well as avoiding potential risks. Ideally, optimal scale-up strategies must be carefully considered and identified during the early stages of development, as making changes later in the bioprocess workflow will require re-optimization and validation, which may have a significant long-term impact on the cost of the therapy. This article provides a summary of important cell culture processing variables to consider in the scale-up of MSC manufacturing as well as giving a comprehensive review of tissue of origin-specific biological characteristics of MSCs and their use in current clinical trials in a range of renal pathologies.
Keywords: advanced therapy medicinal products (ATMPs); cell therapy; clinical application; good manufacturing practice (GMP); kidney disease; mesenchymal stromal cells (MSCs); tissue source.
Copyright © 2021 Calcat-i-Cervera, Sanz-Nogués and O'Brien.
Conflict of interest statement
TO'B is a founder, director and equity holder in Orbsen Therapeutics Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Harmonised culture procedures minimise but do not eliminate mesenchymal stromal cell donor and tissue variability in a decentralised multicentre manufacturing approach.Stem Cell Res Ther. 2023 May 4;14(1):120. doi: 10.1186/s13287-023-03352-1. Stem Cell Res Ther. 2023. PMID: 37143116 Free PMC article.
-
The issue of heterogeneity of MSC-based advanced therapy medicinal products-a review.Front Cell Dev Biol. 2024 Jul 26;12:1400347. doi: 10.3389/fcell.2024.1400347. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39129786 Free PMC article. Review.
-
Systematic review and meta-analysis of cell therapy for COVID-19: global clinical trial landscape, published safety/efficacy outcomes, cell product manufacturing and clinical delivery.Front Immunol. 2023 Jun 21;14:1200180. doi: 10.3389/fimmu.2023.1200180. eCollection 2023. Front Immunol. 2023. PMID: 37415976 Free PMC article.
-
Upstream Process Protocol for MSCs Isolated from Different Human-Based Tissue Origins.Methods Mol Biol. 2024 Jul 6. doi: 10.1007/7651_2024_553. Online ahead of print. Methods Mol Biol. 2024. PMID: 38967911
-
Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome.Front Immunol. 2022 Jun 9;13:918565. doi: 10.3389/fimmu.2022.918565. eCollection 2022. Front Immunol. 2022. PMID: 35812460 Free PMC article. Review.
Cited by
-
The role of MSCs and CAR-MSCs in cellular immunotherapy.Cell Commun Signal. 2023 Aug 1;21(1):187. doi: 10.1186/s12964-023-01191-4. Cell Commun Signal. 2023. PMID: 37528472 Free PMC article. Review.
-
Isolation and Characterization of Canine Adipose-Derived Mesenchymal Stromal Cells: Considerations in Translation from Laboratory to Clinic.Animals (Basel). 2024 Oct 15;14(20):2974. doi: 10.3390/ani14202974. Animals (Basel). 2024. PMID: 39457904 Free PMC article.
-
Mesenchymal stem cells from different sources for sepsis treatment: prospects and limitations.Braz J Med Biol Res. 2024 Oct 14;57:e13457. doi: 10.1590/1414-431X2024e13457. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 39417448 Free PMC article. Review.
-
The Evolving Landscape of Potency Assays.Adv Exp Med Biol. 2023;1420:165-189. doi: 10.1007/978-3-031-30040-0_11. Adv Exp Med Biol. 2023. PMID: 37258790
-
How to Best Protect Kidneys for Transplantation-Mechanistic Target.J Clin Med. 2023 Feb 23;12(5):1787. doi: 10.3390/jcm12051787. J Clin Med. 2023. PMID: 36902572 Free PMC article. Review.
References
-
- WHO . Global Health Estimates. (2019). Available online at: https://www.who.int/data/global-health-estimates (accessed July 19, 2021).
-
- GBD Chronic Kidney Disease Collaboration: Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395:709–33. 10.1016/s0140-6736(20)30045-3 - DOI - PMC - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney. (2013) 3:1–150. 10.1038/kisup.2012.73 - DOI
Publication types
LinkOut - more resources
Full Text Sources

