Efficacy of Immune Checkpoint Inhibitors in Rare Tumours: A Systematic Review
- PMID: 34616395
- PMCID: PMC8488393
- DOI: 10.3389/fimmu.2021.720748
Efficacy of Immune Checkpoint Inhibitors in Rare Tumours: A Systematic Review
Abstract
Background: Rare cancers, as defined by the European Union, occur in fewer than 15 out of 100,000 people each year. The International Rare Cancer Consortium defines rare cancer incidence as less than six per 100,000 per year. There is a growing number of reports of the efficacy of immune checkpoint inhibitor (ICI) therapy in patients with rare tumours, and hence, we conducted a comprehensive review to summarise and analyse the available literature.
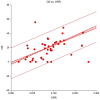
Methods: A literature search of PubMed was performed on January 31, 2021, using the following ICI names as keywords: ipilimumab, tremelimumab, cemiplimab, nivolumab, pembrolizumab, avelumab, atezolizumab, and durvalumab. Studies on patients with rare tumours who were being treated with ICIs were included. We plotted the overall response rate against the corresponding median survival across a variety of cancer types using linear regression.
Results: From 1,255 publications retrieved during the primary search, 62 publications were selected (with a total of 4,620 patients). Only four were randomised trials. A minority were first-line studies, while the remaining were studies in which ICIs were delivered as salvage therapy in pretreated patients. There was a good correlation between response rate and overall survival (Spearman R2 >0.9) in skin cancers, mesothelioma, and sarcomas.
Conclusions: Treatment of advanced-stage rare tumours with ICI therapy was found to be associated with significant activity in some orphan diseases (e.g., Merkel cell carcinoma) and hepatocellular carcinoma. Several ongoing prospective clinical trials will expand the knowledge on the safety and efficacy of ICI therapy in patients with these rare cancers.
Keywords: anti-PD-(L)1 agents; immunotherapy; rare tumours; survival; systematic review.
Copyright © 2021 Petrelli, Consoli, Ghidini, Perego, Luciani, Mercurio, Berruti and Grisanti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Editorial: Dynamic Biomarkers of Response to Anti-Immune Checkpoint Inhibitors in Cancer.Front Immunol. 2021 Oct 21;12:781872. doi: 10.3389/fimmu.2021.781872. eCollection 2021. Front Immunol. 2021. PMID: 34745152 Free PMC article. No abstract available.
-
Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients.Thorac Cancer. 2020 Sep;11(9):2406-2430. doi: 10.1111/1759-7714.13541. Epub 2020 Jul 8. Thorac Cancer. 2020. PMID: 32643323 Free PMC article.
-
Safety and Efficacy of Neoadjuvant Immune Checkpoint Inhibitor Therapy in Patients with Resectable Non-small-Cell Lung Cancer: A Systematic Review.Target Oncol. 2021 Jul;16(4):425-434. doi: 10.1007/s11523-021-00818-1. Epub 2021 May 13. Target Oncol. 2021. PMID: 33983556
-
Application of Immune Checkpoint Inhibitors in the Treatment of Cholangiocarcinoma.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211039952. doi: 10.1177/15330338211039952. Technol Cancer Res Treat. 2021. PMID: 34528830 Free PMC article. Review.
-
Predictive Biomarkers of Immune Checkpoint Inhibitors-Related Toxicities.Front Immunol. 2020 Oct 6;11:2023. doi: 10.3389/fimmu.2020.02023. eCollection 2020. Front Immunol. 2020. PMID: 33123120 Free PMC article. Review.
Cited by
-
Nivolumab plus ipilimumab with chemotherapy in metastatic NSCLC: minireview and a case study of a patient negative for PD-L1.Drugs Context. 2024 Jul 19;13:2024-5-3. doi: 10.7573/dic.2024-5-3. eCollection 2024. Drugs Context. 2024. PMID: 39072302 Free PMC article.
-
NcRNAs: Multi‑angle participation in the regulation of glioma chemotherapy resistance (Review).Int J Oncol. 2022 Jun;60(6):76. doi: 10.3892/ijo.2022.5366. Epub 2022 May 4. Int J Oncol. 2022. PMID: 35506469 Free PMC article. Review.
-
Bias and inconsistency in the estimation of tumour mutation burden.BMC Cancer. 2022 Aug 2;22(1):840. doi: 10.1186/s12885-022-09897-3. BMC Cancer. 2022. PMID: 35918650 Free PMC article.
-
Descriptive Epidemiology of Hospitalization of Patients with a Rare Tumor in an Italian Region.Curr Oncol. 2022 Dec 8;29(12):9711-9721. doi: 10.3390/curroncol29120762. Curr Oncol. 2022. PMID: 36547176 Free PMC article.
-
The Efficacy and Safety of Immune Checkpoint Inhibitors in Adrenocortical Carcinoma: A Systematic Review and Meta-Analysis.Cancers (Basel). 2024 Feb 23;16(5):900. doi: 10.3390/cancers16050900. Cancers (Basel). 2024. PMID: 38473262 Free PMC article. Review.
References
-
- Tamura K, Hasegawa K, Katsumata N, Matsumoto K, Mukai H, Takahashi S, et al. . Efficacy and Safety of Nivolumab in Japanese Patients With Uterine Cervical Cancer, Uterine Corpus Cancer, or Soft Tissue Sarcoma: Multicenter, Open-Label Phase 2 Trial. Cancer Sci (2019) 110(9):2894–904. doi: 10.1111/cas.14148 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources