Maternal gut microbiome regulates immunity to RSV infection in offspring
- PMID: 34613328
- PMCID: PMC8500238
- DOI: 10.1084/jem.20210235
Maternal gut microbiome regulates immunity to RSV infection in offspring
Abstract
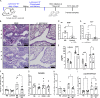
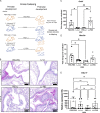
Development of the immune system can be influenced by diverse extrinsic and intrinsic factors that influence the risk of disease. Severe early life respiratory syncytial virus (RSV) infection is associated with persistent immune alterations. Previously, our group had shown that adult mice orally supplemented with Lactobacillus johnsonii exhibited decreased airway immunopathology following RSV infection. Here, we demonstrate that offspring of mice supplemented with L. johnsonii exhibit reduced airway mucus and Th2 cell-mediated response to RSV infection. Maternal supplementation resulted in a consistent gut microbiome in mothers and their offspring. Importantly, supplemented maternal plasma and breastmilk, and offspring plasma, exhibited decreased inflammatory metabolites. Cross-fostering studies showed that prenatal Lactobacillus exposure led to decreased Th2 cytokines and lung inflammation following RSV infection, while postnatal Lactobacillus exposure diminished goblet cell hypertrophy and mucus production in the lung in response to airway infection. These studies demonstrate that Lactobacillus modulation of the maternal microbiome and associated metabolic reprogramming enhance airway protection against RSV in neonates.
© 2021 Fonseca et al.
Conflict of interest statement
Disclosures: S.V. Lynch reported personal fees from Siolta Therapeutics Inc. during the conduct of the study and outside the submitted work; in addition, S.V. Lynch had a patent to Reductive prodrug cancer chemothera (Stan449-PRV) issued, a patent to combination antibiotic and antibody therapy for the treatment of Pseudomonas aeruginosa infection (WO 2010091189 A1) issued, a patent to therapeutic microbial consortium for induction of immune tolerance with royalties paid to Siolta Therapeutics Inc., a patent to systems and methods for detecting antibiotic resistance (WO 2012027302 A3) issued, a patent to nitroreductase enzymes (US 7687474 B2) issued, a patent to sinusitis diagnostics and treatments (WO 2013155370 A1) issued, a patent to methods and systems for phylogenetic analysis (US 20120264637 A1) issued, a patent to methods and compositions relating to epoxide hydrolase genes licensed to Siolta Therapeutics Inc., and a patent to novel Lactobacillus and Micrococcus species that promote tolerogenic immunity pending. No other disclosures were reported.
Figures





Similar articles
-
Lactobacillus johnsonii supplementation attenuates respiratory viral infection via metabolic reprogramming and immune cell modulation.Mucosal Immunol. 2017 Nov;10(6):1569-1580. doi: 10.1038/mi.2017.13. Epub 2017 Mar 15. Mucosal Immunol. 2017. PMID: 28295020 Free PMC article.
-
NLRP3-Inflammasome Inhibition during Respiratory Virus Infection Abrogates Lung Immunopathology and Long-Term Airway Disease Development.Viruses. 2021 Apr 16;13(4):692. doi: 10.3390/v13040692. Viruses. 2021. PMID: 33923693 Free PMC article.
-
Prefusion RSV F Immunization Elicits Th2-Mediated Lung Pathology in Mice When Formulated With a Th2 (but Not a Th1/Th2-Balanced) Adjuvant Despite Complete Viral Protection.Front Immunol. 2020 Jul 29;11:1673. doi: 10.3389/fimmu.2020.01673. eCollection 2020. Front Immunol. 2020. PMID: 32849580 Free PMC article.
-
Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease.Immunol Res. 2007;39(1-3):225-39. doi: 10.1007/s12026-007-0071-6. Immunol Res. 2007. PMID: 17917067 Review.
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
Cited by
-
Complete Genome Sequence of Lactobacillus johnsonii MR1, Isolated from a BALB/c Mouse Cecum.Microbiol Resour Announc. 2023 Jan 24;12(1):e0107822. doi: 10.1128/mra.01078-22. Epub 2022 Dec 13. Microbiol Resour Announc. 2023. PMID: 36511660 Free PMC article.
-
Early-life risk factors which govern pro-allergic immunity.Semin Immunopathol. 2024 Jul 27;46(3-4):9. doi: 10.1007/s00281-024-01020-x. Semin Immunopathol. 2024. PMID: 39066790 Free PMC article. Review.
-
Microbiome-Immune Interactions in Allergy and Asthma.J Allergy Clin Immunol Pract. 2022 Sep;10(9):2244-2251. doi: 10.1016/j.jaip.2022.05.038. Epub 2022 Jun 18. J Allergy Clin Immunol Pract. 2022. PMID: 35724951 Free PMC article. Review.
-
Editorial: Role of lung and gut microbiota in the immune response against respiratory viral infections.Front Immunol. 2023 Jan 9;13:1114581. doi: 10.3389/fimmu.2022.1114581. eCollection 2022. Front Immunol. 2023. PMID: 36700207 Free PMC article. No abstract available.
-
Early-Life Lung and Gut Microbiota Development and Respiratory Syncytial Virus Infection.Front Immunol. 2022 Apr 4;13:877771. doi: 10.3389/fimmu.2022.877771. eCollection 2022. Front Immunol. 2022. PMID: 35444639 Free PMC article. Review.
References
-
- Bergström, A., Skov T.H., Bahl M.I., Roager H.M., Christensen L.B., Ejlerskov K.T., Mølgaard C., Michaelsen K.F., and Licht T.R.. 2014. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 80:2889–2900. 10.1128/AEM.00342-14 - DOI - PMC - PubMed
-
- Borthakur, A., Saksena S., Gill R.K., Alrefai W.A., Ramaswamy K., and Dudeja P.K.. 2008. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-kappaB pathway. J. Cell. Biochem. 103:1452–1463. 10.1002/jcb.21532 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

