Divergent acyl carrier protein decouples mitochondrial Fe-S cluster biogenesis from fatty acid synthesis in malaria parasites
- PMID: 34612205
- PMCID: PMC8547962
- DOI: 10.7554/eLife.71636
Divergent acyl carrier protein decouples mitochondrial Fe-S cluster biogenesis from fatty acid synthesis in malaria parasites
Abstract
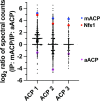
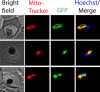
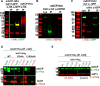
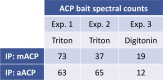
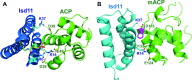
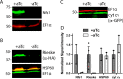
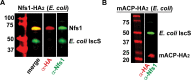
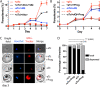
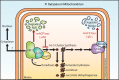
Most eukaryotic cells retain a mitochondrial fatty acid synthesis (FASII) pathway whose acyl carrier protein (mACP) and 4-phosphopantetheine (Ppant) prosthetic group provide a soluble scaffold for acyl chain synthesis and biochemically couple FASII activity to mitochondrial electron transport chain (ETC) assembly and Fe-S cluster biogenesis. In contrast, the mitochondrion of Plasmodium falciparum malaria parasites lacks FASII enzymes yet curiously retains a divergent mACP lacking a Ppant group. We report that ligand-dependent knockdown of mACP is lethal to parasites, indicating an essential FASII-independent function. Decyl-ubiquinone rescues parasites temporarily from death, suggesting a dominant dysfunction of the mitochondrial ETC. Biochemical studies reveal that Plasmodium mACP binds and stabilizes the Isd11-Nfs1 complex required for Fe-S cluster biosynthesis, despite lacking the Ppant group required for this association in other eukaryotes, and knockdown of parasite mACP causes loss of Nfs1 and the Rieske Fe-S protein in ETC complex III. This work reveals that Plasmodium parasites have evolved to decouple mitochondrial Fe-S cluster biogenesis from FASII activity, and this adaptation is a shared metabolic feature of other apicomplexan pathogens, including Toxoplasma and Babesia. This discovery unveils an evolutionary driving force to retain interaction of mitochondrial Fe-S cluster biogenesis with ACP independent of its eponymous function in FASII.
Keywords: Fe-S cluster synthesis; P. falciparum; acyl carrier protein; biochemistry; chemical biology; infectious disease; malaria; microbiology; mitochondria; organelle adaptation.
© 2021, Falekun et al.
Conflict of interest statement
SF, JS, YJ, HP, JW, PS No competing interests declared
Figures








Similar articles
-
The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis.Elife. 2016 Aug 19;5:e17828. doi: 10.7554/eLife.17828. Elife. 2016. PMID: 27540631 Free PMC article.
-
Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):E5325-E5334. doi: 10.1073/pnas.1702849114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634302 Free PMC article.
-
A key cytosolic iron-sulfur cluster synthesis protein localizes to the mitochondrion of Toxoplasma gondii.Mol Microbiol. 2021 May;115(5):968-985. doi: 10.1111/mmi.14651. Epub 2020 Dec 13. Mol Microbiol. 2021. PMID: 33222310
-
Mitochondrial acyl carrier protein (ACP) at the interface of metabolic state sensing and mitochondrial function.Biochim Biophys Acta Mol Cell Res. 2019 Dec;1866(12):118540. doi: 10.1016/j.bbamcr.2019.118540. Epub 2019 Aug 29. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31473256 Review.
-
Iron-sulphur clusters, their biosynthesis, and biological functions in protozoan parasites.Adv Parasitol. 2013;83:1-92. doi: 10.1016/B978-0-12-407705-8.00001-X. Adv Parasitol. 2013. PMID: 23876871 Review.
Cited by
-
Direct Tests of Cytochrome Function in the Electron Transport Chain of Malaria Parasites.bioRxiv [Preprint]. 2023 Jan 23:2023.01.23.525242. doi: 10.1101/2023.01.23.525242. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2301047120. doi: 10.1073/pnas.2301047120 PMID: 36747727 Free PMC article. Updated. Preprint.
-
Installation of LYRM proteins in early eukaryotes to regulate the metabolic capacity of the emerging mitochondrion.Open Biol. 2024 May;14(5):240021. doi: 10.1098/rsob.240021. Epub 2024 May 22. Open Biol. 2024. PMID: 38772414 Free PMC article.
-
Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2411631121. doi: 10.1073/pnas.2411631121. Epub 2024 Oct 28. Proc Natl Acad Sci U S A. 2024. PMID: 39467134
-
Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites.bioRxiv [Preprint]. 2024 Jun 10:2024.05.10.587216. doi: 10.1101/2024.05.10.587216. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2411631121. doi: 10.1073/pnas.2411631121 PMID: 38798484 Free PMC article. Updated. Preprint.
-
Direct tests of cytochrome c and c1 functions in the electron transport chain of malaria parasites.Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2301047120. doi: 10.1073/pnas.2301047120. Epub 2023 May 1. Proc Natl Acad Sci U S A. 2023. PMID: 37126705 Free PMC article.
References
-
- Angerer H, Radermacher M, Mańkowska M, Steger M, Zwicker K, Heide H, Wittig I, Brandt U, Zickermann V. The LYR protein subunit NB4M/NDUFA6 of mitochondrial complex I anchors an acyl carrier protein and is essential for catalytic activity. PNAS. 2014;111:5207–5212. doi: 10.1073/pnas.1322438111. - DOI - PMC - PubMed
-
- Antonova-Koch Y, Meister S, Abraham M, Luth MR, Ottilie S, Lukens AK, Sakata-Kato T, Vanaerschot M, Owen E, Jado JC, Maher SP, Calla J, Plouffe D, Zhong Y, Chen K, Chaumeau V, Conway AJ, McNamara CW, Ibanez M, Gagaring K, Serrano FN, Eribez K, Taggard CM, Cheung AL, Lincoln C, Ambachew B, Rouillier M, Siegel D, Nosten F, Kyle DE, Gamo F-J, Zhou Y, Llinás M, Fidock DA, Wirth DF, Burrows J, Campo B, Winzeler EA. Open-source discovery of chemical leads for next-generation chemoprotective antimalarials. Science. 2018;362:eaat9446. doi: 10.1126/science.aat9446. - DOI - PMC - PubMed

