NK and CD8+ T cell phenotypes predict onset and control of CMV viremia after kidney transplant
- PMID: 34609965
- PMCID: PMC8663544
- DOI: 10.1172/jci.insight.153175
NK and CD8+ T cell phenotypes predict onset and control of CMV viremia after kidney transplant
Abstract
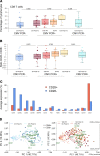
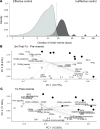
CMV causes mostly asymptomatic but lifelong infection. Primary infection or reactivation in immunocompromised individuals can be life-threatening. CMV viremia often occurs in solid organ transplant recipients and associates with decreased graft survival and higher mortality. Furthering understanding of impaired immunity that allows CMV reactivation is critical to guiding antiviral therapy and examining the effect of CMV on solid organ transplant outcomes. This study characterized longitudinal immune responses to CMV in 31 kidney transplant recipients with CMV viremia and matched, nonviremic recipients. Recipients were sampled 3 and 12 months after transplant, with additional samples 1 week and 1 month after viremia. PBMCs were stained for NK and T cell markers. PBMC transcriptomes were characterized by RNA-Seq. Plasma proteins were quantified by Luminex. CD8+ T cell transcriptomes were characterized by single-cell RNA-Seq. Before viremia, patients had high levels of IL-15 with concurrent expansion of immature CD56bright NK cells. After viremia, mature CD56dim NK cells and CD28-CD8+ T cells upregulating inhibitory and NK-associated receptors were expanded. Memory NK cells and NK-like CD28-CD8+ T cells were associated with control of viremia. These findings suggest that signatures of innate activation may be prognostic for CMV reactivation after transplant, while CD8+ T cell functionality is critical for effective control of CMV.
Keywords: Immunology; NK cells; Organ transplantation; T cells; Transplantation.
Figures



Similar articles
-
Mass cytometry reveals single-cell kinetics of cytotoxic lymphocyte evolution in CMV-infected renal transplant patients.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2116588119. doi: 10.1073/pnas.2116588119. Proc Natl Acad Sci U S A. 2022. PMID: 35181606 Free PMC article.
-
CD28 positive, cytomegalovirus specific cytotoxic T lymphocytes as a novel biomarker associated with cytomegalovirus viremia in kidney allorecipients.J Clin Virol. 2016 Oct;83:17-25. doi: 10.1016/j.jcv.2016.08.290. Epub 2016 Aug 10. J Clin Virol. 2016. PMID: 27526103
-
Regulation of Adaptive NK Cells and CD8 T Cells by HLA-C Correlates with Allogeneic Hematopoietic Cell Transplantation and with Cytomegalovirus Reactivation.J Immunol. 2015 Nov 1;195(9):4524-36. doi: 10.4049/jimmunol.1401990. Epub 2015 Sep 28. J Immunol. 2015. PMID: 26416275 Free PMC article.
-
[Monitoring of cytomegalovirus-specific CD4+ and CD8+ T cell responses by cytokine flow cytometry in renal transplant recipients].Mikrobiyol Bul. 2016 Apr;50(2):224-35. Mikrobiyol Bul. 2016. PMID: 27175495 Turkish.
-
γδ T Cell-Mediated Immunity to Cytomegalovirus Infection.Front Immunol. 2017 Feb 9;8:105. doi: 10.3389/fimmu.2017.00105. eCollection 2017. Front Immunol. 2017. PMID: 28232834 Free PMC article. Review.
Cited by
-
Association of Cytomegalovirus (CMV) DNAemia With Long-Term Mortality in a Randomized Trial of Preemptive Therapy and Antiviral Prophylaxis for Prevention of CMV Disease in High-Risk Donor Seropositive, Recipient Seronegative Liver Transplant Recipients.Clin Infect Dis. 2024 Mar 20;78(3):719-722. doi: 10.1093/cid/ciad643. Clin Infect Dis. 2024. PMID: 37862162 Free PMC article.
-
The advance of single cell transcriptome to study kidney immune cells in diabetic kidney disease.BMC Nephrol. 2024 Nov 16;25(1):412. doi: 10.1186/s12882-024-03853-y. BMC Nephrol. 2024. PMID: 39550562 Free PMC article. Review.
-
T-lymphocyte subtyping: an early warning and a potential prognostic indicator of active cytomegalovirus infection in patients with sepsis.Immunol Cell Biol. 2022 Nov;100(10):777-790. doi: 10.1111/imcb.12586. Epub 2022 Oct 12. Immunol Cell Biol. 2022. PMID: 36106958 Free PMC article.
-
Plac8-ERK pathway modulation of monocyte function in sepsis.Cell Death Discov. 2024 Jul 3;10(1):308. doi: 10.1038/s41420-024-02012-4. Cell Death Discov. 2024. PMID: 38961068 Free PMC article.
-
Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion.J Exp Med. 2022 Aug 1;219(8):e20212434. doi: 10.1084/jem.20212434. Epub 2022 Jun 27. J Exp Med. 2022. PMID: 35758909 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

