Citrobacter amalonaticus Inhibits the Growth of Citrobacter rodentium in the Gut Lumen
- PMID: 34609899
- PMCID: PMC8510533
- DOI: 10.1128/mBio.02410-21
Citrobacter amalonaticus Inhibits the Growth of Citrobacter rodentium in the Gut Lumen
Abstract
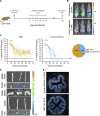
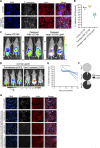
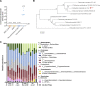
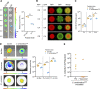
The gut microbiota plays a crucial role in susceptibility to enteric pathogens, including Citrobacter rodentium, a model extracellular mouse pathogen that colonizes the colonic mucosa. C. rodentium infection outcomes vary between mouse strains, with C57BL/6 and C3H/HeN mice clearing and succumbing to the infection, respectively. Kanamycin (Kan) treatment at the peak of C57BL/6 mouse infection with Kan-resistant C. rodentium resulted in relocalization of the pathogen from the colonic mucosa and cecum to solely the cecal luminal contents; cessation of the Kan treatment resulted in rapid clearance of the pathogen. We now show that in C3H/HeN mice, following Kan-induced displacement of C. rodentium to the cecum, the pathogen stably colonizes the cecal lumens of 65% of the mice in the absence of continued antibiotic treatment, a phenomenon that we term antibiotic-induced bacterial commensalization (AIBC). AIBC C. rodentium was well tolerated by the host, which showed few signs of inflammation; passaged AIBC C. rodentium robustly infected naive C3H/HeN mice, suggesting that the AIBC state is transient and did not select for genetically avirulent C. rodentium mutants. Following withdrawal of antibiotic treatment, 35% of C3H/HeN mice were able to prevent C. rodentium commensalization in the gut lumen. These mice presented a bloom of a commensal species, Citrobacter amalonaticus, which inhibited the growth of C. rodentium in vitro in a contact-dependent manner and the luminal growth of AIBC C. rodentium in vivo. Overall, our data suggest that commensal species can confer colonization resistance to closely related pathogenic species. IMPORTANCE Gut bacterial infections involve three-way interactions between virulence factors, the host immune responses, and the microbiome. While the microbiome erects colonization resistance barriers, pathogens employ virulence factors to overcome them. Treating mice infected with kanamycin-resistant Citrobacter rodentium with kanamycin caused displacement of the pathogen from the colonic mucosa to the cecal lumen. Following withdrawal of the kanamycin treatment, 65% of the mice were persistently colonized by C. rodentium, which seemed to downregulate virulence factor expression. In this model of luminal gut colonization, 35% of mice were refractory to stable C. rodentium colonization, suggesting that their microbiotas were able to confer colonization resistance. We identify a commensal bacterium of the Citrobacter genus, C. amalonaticus, which inhibits C. rodentium growth in vitro and in vivo. These results show that the line separating commensal and pathogenic lifestyles is thin and multifactorial and that commensals may play a major role in combating enteric infection.
Keywords: Citrobacter; colonization resistance; gastrointestinal infection.
Figures

Similar articles
-
Citrobacter rodentium Relies on Commensals for Colonization of the Colonic Mucosa.Cell Rep. 2017 Dec 19;21(12):3381-3389. doi: 10.1016/j.celrep.2017.11.086. Cell Rep. 2017. PMID: 29262319 Free PMC article.
-
Intestinal Epithelial Cells and the Microbiome Undergo Swift Reprogramming at the Inception of Colonic Citrobacter rodentium Infection.mBio. 2019 Apr 2;10(2):e00062-19. doi: 10.1128/mBio.00062-19. mBio. 2019. PMID: 30940698 Free PMC article.
-
Overview of the Effect of Citrobacter rodentium Infection on Host Metabolism and the Microbiota.Methods Mol Biol. 2021;2291:399-418. doi: 10.1007/978-1-0716-1339-9_20. Methods Mol Biol. 2021. PMID: 33704766 Review.
-
Hyaluronan-induced alterations of the gut microbiome protects mice against Citrobacter rodentium infection and intestinal inflammation.Gut Microbes. 2021 Jan-Dec;13(1):1972757. doi: 10.1080/19490976.2021.1972757. Gut Microbes. 2021. PMID: 34592891 Free PMC article.
-
Citrobacter rodentium: infection, inflammation and the microbiota.Nat Rev Microbiol. 2014 Sep;12(9):612-23. doi: 10.1038/nrmicro3315. Epub 2014 Aug 4. Nat Rev Microbiol. 2014. PMID: 25088150 Review.
Cited by
-
Efficacy of EHEC gold nanoparticle vaccines evaluated with the Shiga toxin-producing Citrobacter rodentium mouse model.Microbiol Spectr. 2024 Jan 11;12(1):e0226123. doi: 10.1128/spectrum.02261-23. Epub 2023 Dec 4. Microbiol Spectr. 2024. PMID: 38047703 Free PMC article.
-
Cooperation between physiological defenses and immune resistance produces asymptomatic carriage of a lethal bacterial pathogen.bioRxiv [Preprint]. 2023 Feb 26:2023.01.22.525099. doi: 10.1101/2023.01.22.525099. bioRxiv. 2023. Update in: Sci Adv. 2023 Jun 23;9(25):eadg8719. doi: 10.1126/sciadv.adg8719. PMID: 36711884 Free PMC article. Updated. Preprint.
-
Cooperation between physiological defenses and immune resistance produces asymptomatic carriage of a lethal bacterial pathogen.Sci Adv. 2023 Jun 23;9(25):eadg8719. doi: 10.1126/sciadv.adg8719. Epub 2023 Jun 23. Sci Adv. 2023. PMID: 37352357 Free PMC article.
-
Deciphering the gastrointestinal carriage of Klebsiella pneumoniae.Infect Immun. 2024 Sep 10;92(9):e0048223. doi: 10.1128/iai.00482-23. Epub 2024 Apr 10. Infect Immun. 2024. PMID: 38597634 Free PMC article. Review.
-
Microbiota-mediated colonization resistance: mechanisms and regulation.Nat Rev Microbiol. 2023 Jun;21(6):347-360. doi: 10.1038/s41579-022-00833-7. Epub 2022 Dec 20. Nat Rev Microbiol. 2023. PMID: 36539611 Free PMC article. Review.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

