Uncommon Bis-Amide Matrine-type Alkaloids From Sophora alopecuroides With Anti-inflammatory Effects
- PMID: 34604173
- PMCID: PMC8479178
- DOI: 10.3389/fchem.2021.740421
Uncommon Bis-Amide Matrine-type Alkaloids From Sophora alopecuroides With Anti-inflammatory Effects
Abstract
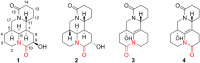
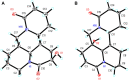
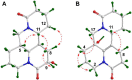
Four new alkaloids (1-4) belonging to rare examples of bis-amide matrine-type were isolated from the seeds of sophora alopecuroides. Their structures including absolute configuration were determined by extensive spectroscopic analysis, electronic circular dichroism (ECD) interpretation, and X-ray diffraction crystallography. Chemically, bis-amide matrine-type alkaloids can provide new molecular template for structural modification. Compounds 3-4 displayed obvious anti-inflammatory effects based on the inhibition of two key pro-inflammatory cytokines [tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)] in a dose-dependent manner, with IC50 values from 35.6 to 45.8 μm.
Keywords: Sophora alopecuroides; matrine-type alkaloids; structure elucidation; structure modification; water-soluble alkaloids.
Copyright © 2021 Luo, Tu, Yin, Fan, Chen, Wu, Ding, Li, Wang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
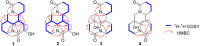

Similar articles
-
Water-soluble matrine-type alkaloids with potential anti-neuroinflammatory activities from the seeds of Sophora alopecuroides.Bioorg Chem. 2021 Nov;116:105337. doi: 10.1016/j.bioorg.2021.105337. Epub 2021 Sep 11. Bioorg Chem. 2021. PMID: 34521046
-
Undescribed matrine-type alkaloids from Sophora alopecuroides with anti-inflammatory activity.Phytochemistry. 2024 Feb;218:113954. doi: 10.1016/j.phytochem.2023.113954. Epub 2023 Dec 16. Phytochemistry. 2024. PMID: 38104747
-
Matrine-Type Alkaloids from the Seeds of Sophora alopecuroides and Their Potential Anti-inflammatory Activities.Chem Biodivers. 2021 Apr;18(4):e2001066. doi: 10.1002/cbdv.202001066. Epub 2021 Mar 3. Chem Biodivers. 2021. PMID: 33656782
-
Quinolizidine alkaloids derivatives from Sophora alopecuroides Linn: Bioactivities, structure-activity relationships and preliminary molecular mechanisms.Eur J Med Chem. 2020 Feb 15;188:111972. doi: 10.1016/j.ejmech.2019.111972. Epub 2019 Dec 23. Eur J Med Chem. 2020. PMID: 31884408 Review.
-
Research Advances on Matrine.Front Chem. 2022 Apr 1;10:867318. doi: 10.3389/fchem.2022.867318. eCollection 2022. Front Chem. 2022. PMID: 35433636 Free PMC article. Review.
Cited by
-
Effects of Sophora alopecuroides in a High-Concentrate Diet on the Liver Immunity and Antioxidant Function of Lambs According to Transcriptome Analysis.Animals (Basel). 2024 Jan 5;14(2):182. doi: 10.3390/ani14020182. Animals (Basel). 2024. PMID: 38254353 Free PMC article.
-
Research Progress of Natural Matrine Compounds and Synthetic Matrine Derivatives.Molecules. 2023 Jul 31;28(15):5780. doi: 10.3390/molecules28155780. Molecules. 2023. PMID: 37570750 Free PMC article. Review.
-
Exploring the Potential of Natural Product-Based Nanomedicine for Maintaining Oral Health.Molecules. 2022 Mar 7;27(5):1725. doi: 10.3390/molecules27051725. Molecules. 2022. PMID: 35268826 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

