Cryo-electron microscopy of nodavirus RNA replication organelles illuminates positive-strand RNA virus genome replication
- PMID: 34601307
- PMCID: PMC8504867
- DOI: 10.1016/j.coviro.2021.09.008
Cryo-electron microscopy of nodavirus RNA replication organelles illuminates positive-strand RNA virus genome replication
Abstract
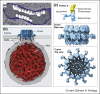
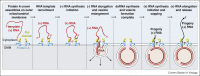
The nodavirus flock house virus recently provided a well-characterized model for the first cryo-electron microscope tomography of membrane-bound, positive-strand RNA ((+)RNA) virus genome replication complexes (RCs). The resulting first views of RC organization and complementary biochemical results showed that the viral RNA replication vesicle is tightly packed with the dsRNA genomic RNA replication intermediate, and that (+)ssRNA replication products are released through the vesicle neck to the cytosol through a 12-fold symmetric ring or crown of multi-functional viral RNA replication proteins, which likely also contribute to viral RNA synthesis. Subsequent studies identified similar crown-like RNA replication protein complexes in alphavirus and coronavirus RCs, indicating related mechanisms across highly divergent (+)RNA viruses. As outlined in this review, these results have significant implications for viral function, evolution and control.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Multifunctional Protein A Is the Only Viral Protein Required for Nodavirus RNA Replication Crown Formation.Viruses. 2022 Dec 3;14(12):2711. doi: 10.3390/v14122711. Viruses. 2022. PMID: 36560715 Free PMC article.
-
Subdomain cryo-EM structure of nodaviral replication protein A crown complex provides mechanistic insights into RNA genome replication.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18680-18691. doi: 10.1073/pnas.2006165117. Epub 2020 Jul 20. Proc Natl Acad Sci U S A. 2020. PMID: 32690711 Free PMC article.
-
Cryo-electron tomography reveals novel features of a viral RNA replication compartment.Elife. 2017 Jun 27;6:e25940. doi: 10.7554/eLife.25940. Elife. 2017. PMID: 28653620 Free PMC article.
-
Positive-strand RNA virus genome replication organelles: structure, assembly, control.Trends Genet. 2024 Aug;40(8):681-693. doi: 10.1016/j.tig.2024.04.003. Epub 2024 May 8. Trends Genet. 2024. PMID: 38724328 Review.
-
Crowning Touches in Positive-Strand RNA Virus Genome Replication Complex Structure and Function.Annu Rev Virol. 2022 Sep 29;9(1):193-212. doi: 10.1146/annurev-virology-092920-021307. Epub 2022 May 24. Annu Rev Virol. 2022. PMID: 35610038 Review.
Cited by
-
Deciphering the interaction surface between the West Nile virus NS3 and NS5 proteins.Access Microbiol. 2024 Jun 26;6(6):000675.v3. doi: 10.1099/acmi.0.000675.v3. eCollection 2024. Access Microbiol. 2024. PMID: 39045235 Free PMC article.
-
Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy.Molecules. 2023 Nov 20;28(22):7679. doi: 10.3390/molecules28227679. Molecules. 2023. PMID: 38005401 Free PMC article. Review.
-
Nodavirus RNA replication crown architecture reveals proto-crown precursor and viral protein A conformational switching.Proc Natl Acad Sci U S A. 2023 Jan 31;120(5):e2217412120. doi: 10.1073/pnas.2217412120. Epub 2023 Jan 24. Proc Natl Acad Sci U S A. 2023. PMID: 36693094 Free PMC article.
-
Multifunctional Protein A Is the Only Viral Protein Required for Nodavirus RNA Replication Crown Formation.Viruses. 2022 Dec 3;14(12):2711. doi: 10.3390/v14122711. Viruses. 2022. PMID: 36560715 Free PMC article.
-
Molecular architecture of the Chikungunya virus replication complex.Sci Adv. 2022 Dec 2;8(48):eadd2536. doi: 10.1126/sciadv.add2536. Epub 2022 Nov 30. Sci Adv. 2022. PMID: 36449616 Free PMC article.
References
-
- Unchwaniwala N., Ahlquist P. Coronavirus dons a new crown. Science. 2020;69:1306–1307. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

