Pituitary Adenylate Cyclase Activating Polypeptide Inhibits A10 Dopamine Neurons and Suppresses the Binge-like Consumption of Palatable Food
- PMID: 34597709
- PMCID: PMC8608708
- DOI: 10.1016/j.neuroscience.2021.09.016
Pituitary Adenylate Cyclase Activating Polypeptide Inhibits A10 Dopamine Neurons and Suppresses the Binge-like Consumption of Palatable Food
Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) binds to PACAP-specific (PAC1) receptors in multiple hypothalamic areas, especially those regulating energy balance. PACAP neurons in the ventromedial nucleus (VMN) exert anorexigenic effects within the homeostatic energy balance circuitry. Since PACAP can also reduce the consumption of palatable food, we tested the hypothesis that VMN PACAP neurons project to the ventral tegmental area (VTA) to inhibit A10 dopamine neurons via PAC1 receptors and KATP channels, and thereby suppress binge-like consumption. We performed electrophysiological recordings in mesencephalic slices from male PACAP-Cre and tyrosine hydroxylase (TH)-Cre mice. Initially, we injected PACAP (30 pmol) into the VTA, where it suppressed binge intake in wildtype male but not female mice. Subsequent tract tracing studies uncovered projections of VMN PACAP neurons to the VTA. Optogenetic stimulation of VMN PACAP neurons in voltage clamp induced an outward current and increase in conductance in VTA neurons, and a hyperpolarization and decrease in firing in current clamp. These effects were markedly attenuated by the KATP channel blocker tolbutamide (100 μM) and PAC1 receptor antagonist PACAP6-38 (200 nM). In recordings from A10 dopamine neurons in TH-Cre mice, we replicated the outward current by perfusing PACAP1-38 (100 nM). This response was again completely blocked by tolbutamide and PACAP6-38, and associated with a hyperpolarization and decrease in firing. These findings demonstrate that PACAP activates PAC1 receptors and KATP channels to inhibit A10 dopamine neurons and sex-dependently suppress binge-like consumption. Accordingly, they advance our understanding of how PACAP regulates energy homeostasis via the hedonic energy balance circuitry.
Keywords: binge eating; dopamine; estradiol; obesity; pituitary adenylate cyclase-activating polypeptide; sex difference.
Copyright © 2021 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



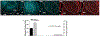

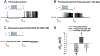
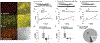
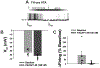
Similar articles
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
-
The role of pituitary adenylate cyclase-activating polypeptide neurons in the hypothalamic ventromedial nucleus and the cognate PAC1 receptor in the regulation of hedonic feeding.Front Nutr. 2024 Aug 21;11:1437526. doi: 10.3389/fnut.2024.1437526. eCollection 2024. Front Nutr. 2024. PMID: 39234295 Free PMC article.
-
Pituitary Adenylate Cyclase-Activating Polypeptide Excites Proopiomelanocortin Neurons: Implications for the Regulation of Energy Homeostasis.Neuroendocrinology. 2021;111(1-2):45-69. doi: 10.1159/000506367. Epub 2020 Feb 7. Neuroendocrinology. 2021. PMID: 32028278
-
Dynamic, sex- and diet-specific pleiotropism in the PAC1 receptor-mediated regulation of arcuate proopiomelanocortin and Neuropeptide Y/Agouti related peptide neuronal excitability by anorexigenic ventromedial nucleus PACAP neurons.J Neuroendocrinol. 2024 Jan;36(1):e13357. doi: 10.1111/jne.13357. Epub 2023 Dec 6. J Neuroendocrinol. 2024. PMID: 38056947
-
[Physiological significance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the nervous system].Yakugaku Zasshi. 2002 Dec;122(12):1109-21. doi: 10.1248/yakushi.122.1109. Yakugaku Zasshi. 2002. PMID: 12510388 Review. Japanese.
Cited by
-
Pituitary adenylate cyclase-activating polypeptide (PACAP) in the paraventricular nucleus of the thalamus: Influence on binge-type eating in male and female mice.Res Sq [Preprint]. 2024 Apr 2:rs.3.rs-4145128. doi: 10.21203/rs.3.rs-4145128/v1. Res Sq. 2024. Update in: Psychopharmacology (Berl). 2025 Feb;242(2):413-426. doi: 10.1007/s00213-024-06692-9. PMID: 38645077 Free PMC article. Updated. Preprint.
-
Pituitary adenylate cyclase-activating polypeptide (PACAP)+ cells in the paraventricular nucleus of the thalamus: relationship with binge-type eating in male and female mice.Psychopharmacology (Berl). 2025 Feb;242(2):413-426. doi: 10.1007/s00213-024-06692-9. Epub 2024 Sep 28. Psychopharmacology (Berl). 2025. PMID: 39340653 Free PMC article.
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
-
Peptidergic and functional delineation of the Edinger-Westphal nucleus.Cell Rep. 2023 Aug 29;42(8):112992. doi: 10.1016/j.celrep.2023.112992. Epub 2023 Aug 17. Cell Rep. 2023. PMID: 37594894 Free PMC article.
-
The vital role of arcuate nociceptin/orphanin FQ neurones in mounting an oestradiol-dependent adaptive response to negative energy balance via inhibition of nearby proopiomelanocortin neurones.J Physiol. 2022 Nov;600(22):4939-4961. doi: 10.1113/JP283378. Epub 2022 Oct 28. J Physiol. 2022. PMID: 36217719 Free PMC article.
References
-
- Bassareo V, Di Chiara G (1999), Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci 11:4389–4397. - PubMed
-
- Beaulieu-Boire I, Lang AE (2015), Behavioral effects of levodopa. Mov Disord 30:90–102. - PubMed
-
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A (2003), Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol 284:R1260–R1268. - PubMed
-
- Berridge KC (1996), Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20:1–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

