The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus
- PMID: 34593848
- PMCID: PMC8484654
- DOI: 10.1038/s41598-021-97797-0
The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus
Abstract
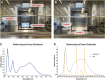
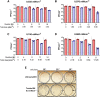
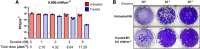
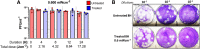
The germicidal potential of specific wavelengths within the electromagnetic spectrum is an area of growing interest. While ultra-violet (UV) based technologies have shown satisfactory virucidal potential, the photo-toxicity in humans coupled with UV associated polymer degradation limit their use in occupied spaces. Alternatively, longer wavelengths with less irradiation energy such as visible light (405 nm) have largely been explored in the context of bactericidal and fungicidal applications. Such studies indicated that 405 nm mediated inactivation is caused by the absorbance of porphyrins within the organism creating reactive oxygen species which result in free radical damage to its DNA and disruption of cellular functions. The virucidal potential of visible-light based technologies has been largely unexplored and speculated to be ineffective given the lack of porphyrins in viruses. The current study demonstrated increased susceptibility of lipid-enveloped respiratory pathogens of importance such as SARS-CoV-2 (causative agent of COVID-19) and influenza A virus to 405 nm, visible light in the absence of exogenous photosensitizers thereby indicating a potential alternative porphyrin-independent mechanism of visible light mediated viral inactivation. These results were obtained using less than expected irradiance levels which are considered safe for humans and commercially achievable. Our results support further exploration of the use of visible light technology for the application of continuous decontamination in occupied areas within hospitals and/or infectious disease laboratories, specifically for the inactivation of respiratory pathogens such as SARS-CoV-2 and Influenza A.
© 2021. The Author(s).
Conflict of interest statement
The García-Sastre Laboratory has received research support from Pfizer, Senhwa Biosciences, 7Hills Pharma, Avimex, Blade Therapeutics, Dynavax, ImmunityBio, Nanocomposix and Kenall Manufacturing. Adolfo García-Sastre has consulting agreements for the following companies involving cash and/or stock: Vivaldi Biosciences, Pagoda, Contrafect, 7Hills Pharma, Avimex, Vaxalto, Accurius, Pfizer and Esperovax. RR, CY and AGS have filed for a provisional patent based upon these results.
Figures
Similar articles
-
UV Inactivation of SARS-CoV-2 across the UVC Spectrum: KrCl* Excimer, Mercury-Vapor, and Light-Emitting-Diode (LED) Sources.Appl Environ Microbiol. 2021 Oct 28;87(22):e0153221. doi: 10.1128/AEM.01532-21. Epub 2021 Sep 8. Appl Environ Microbiol. 2021. PMID: 34495736 Free PMC article.
-
Virucidal Efficacy of Blue LED and Far-UVC Light Disinfection against Feline Infectious Peritonitis Virus as a Model for SARS-CoV-2.Viruses. 2021 Jul 23;13(8):1436. doi: 10.3390/v13081436. Viruses. 2021. PMID: 34452302 Free PMC article.
-
Spectrum of virucidal activity from ultraviolet to infrared radiation.Photochem Photobiol Sci. 2020 Oct 14;19(10):1262-1270. doi: 10.1039/d0pp00221f. Photochem Photobiol Sci. 2020. PMID: 32812619 Free PMC article.
-
Ozone Generation by Ultraviolet Lamps†.Photochem Photobiol. 2021 May;97(3):471-476. doi: 10.1111/php.13391. Epub 2021 Feb 22. Photochem Photobiol. 2021. PMID: 33534912 Review.
-
Air Disinfection for Airborne Infection Control with a Focus on COVID-19: Why Germicidal UV is Essential†.Photochem Photobiol. 2021 May;97(3):493-497. doi: 10.1111/php.13421. Epub 2021 Apr 5. Photochem Photobiol. 2021. PMID: 33759191 Free PMC article. Review.
Cited by
-
Impacts of Surface Characteristics and Dew Point on the Blue-Light (BL405) Inactivation of Viruses.Microorganisms. 2023 Oct 26;11(11):2638. doi: 10.3390/microorganisms11112638. Microorganisms. 2023. PMID: 38004651 Free PMC article.
-
Influence of Visible Violet, Blue and Red Light on the Development of Cataract in Porcine Lenses.Medicina (Kaunas). 2022 May 27;58(6):721. doi: 10.3390/medicina58060721. Medicina (Kaunas). 2022. PMID: 35743984 Free PMC article.
-
The potential role of violet-blue light to preventing hospital acquired infections: a systematic review.Front Public Health. 2024 Oct 24;12:1474295. doi: 10.3389/fpubh.2024.1474295. eCollection 2024. Front Public Health. 2024. PMID: 39512717 Free PMC article.
-
Visible 405 nm Violet-Blue Light Successfully Inactivates HIV-1 in Human Plasma.Pathogens. 2022 Jul 8;11(7):778. doi: 10.3390/pathogens11070778. Pathogens. 2022. PMID: 35890023 Free PMC article.
-
Stability of SARS-CoV-2 in cold-chain transportation environments and the efficacy of disinfection measures.Front Cell Infect Microbiol. 2023 Apr 19;13:1170505. doi: 10.3389/fcimb.2023.1170505. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37153150 Free PMC article.
References
-
- Worldometer, D. COVID-19 coronavirus pandemic. http://www.worldometers.info (World Health Organization, 2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

