Supranutritional selenium suppresses ROS-induced generation of RANKL-expressing osteoclastogenic CD4+ T cells and ameliorates rheumatoid arthritis
- PMID: 34584694
- PMCID: PMC8452973
- DOI: 10.1002/cti2.1338
Supranutritional selenium suppresses ROS-induced generation of RANKL-expressing osteoclastogenic CD4+ T cells and ameliorates rheumatoid arthritis
Abstract
Objective: The benefit of Se supplementation in rheumatoid arthritis (RA) has been tested in clinical trials, but results remain inconclusive. The objective of this study was to specifically investigate the potential benefit of supranutritional Se by examining human samples from an area with supranutritional Se intake and testing a mouse model of RA.
Methods: Peripheral blood mononuclear cells (PBMCs) from RA patients (N = 57) and healthy controls (HC, N = 71) from an area of supranutritional Se intake (Enshi, Hubei, China) were analysed by flow cytometry. Serum cytokine and Se levels were measured by cytometric beads array (CBA) and inductively coupled plasma mass spectrometry (ICP-MS), respectively. With sufficient or supranutritional selenium intake, mice were induced with collagen-induced arthritis (CIA) and examined for disease activity and immunopathology. The influence of Se supplementation in the generation of RANKL-expressing osteoclastogenic CD4+ T cells was investigated by in vitro assays.
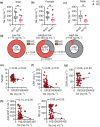
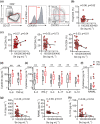
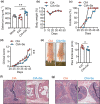
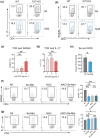
Results: In Enshi city, HC showed the above-normal concentrations of serum Se concentrations while RA patients were enriched in the normal range (70-150 ng mL-1) or below. RA patients with higher Se levels demonstrated milder disease and lower levels of C-reactive protein, IL-6, RANKL and Th17 cells. In the mouse CIA model, supranutritional Se supplementation delayed disease onset, ameliorated joint pathology and reduced CD4+CD44+RANKL+ T cells. Se supplementation could suppress RANKL expression in cultured mouse Th17 cells.
Conclusion: Supranutritional Se suppresses RANKL-expressing osteoclastogenic CD4+ T cells and could be beneficial to RA, which warrants formal testing in randomised clinical trials.
Keywords: RANKL; Th17 cells; rheumatoid arthritis; selenium.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis.Arthritis Rheum. 2012 Mar;64(3):740-51. doi: 10.1002/art.33390. Arthritis Rheum. 2012. PMID: 21968544
-
IL-18 binding protein suppresses IL-17-induced osteoclastogenesis and rectifies type 17 helper T cell / regulatory T cell imbalance in rheumatoid arthritis.J Transl Med. 2021 Sep 16;19(1):392. doi: 10.1186/s12967-021-03071-2. J Transl Med. 2021. PMID: 34530864 Free PMC article.
-
DJ-1 controls T cell differentiation and osteoclastogenesis in rheumatoid arthritis.Sci Rep. 2022 Jul 27;12(1):12767. doi: 10.1038/s41598-022-16285-1. Sci Rep. 2022. PMID: 35896699 Free PMC article.
-
Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis.Bone. 2002 Feb;30(2):340-6. doi: 10.1016/s8756-3282(01)00682-2. Bone. 2002. PMID: 11856640 Review.
-
Bone destruction in arthritis.Ann Rheum Dis. 2002 Nov;61 Suppl 2(Suppl 2):ii84-6. doi: 10.1136/ard.61.suppl_2.ii84. Ann Rheum Dis. 2002. PMID: 12379632 Free PMC article. Review.
Cited by
-
The Role of Selenium in Pathologies: An Updated Review.Antioxidants (Basel). 2022 Jan 27;11(2):251. doi: 10.3390/antiox11020251. Antioxidants (Basel). 2022. PMID: 35204134 Free PMC article. Review.
-
Development and validation of a selenium metabolism regulators associated prognostic model for hepatocellular carcinoma.BMC Cancer. 2023 May 18;23(1):451. doi: 10.1186/s12885-023-10944-w. BMC Cancer. 2023. PMID: 37202783 Free PMC article.
-
Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs.Front Immunol. 2023 Feb 9;14:1107670. doi: 10.3389/fimmu.2023.1107670. eCollection 2023. Front Immunol. 2023. PMID: 36845127 Free PMC article. Review.
-
DNA Methylation of T Lymphocytes as a Therapeutic Target: Implications for Rheumatoid Arthritis Etiology.Front Immunol. 2022 Mar 3;13:863703. doi: 10.3389/fimmu.2022.863703. eCollection 2022. Front Immunol. 2022. PMID: 35309322 Free PMC article. Review.
-
Systemic immunometabolism and responses to vaccines: insights from T and B cell perspectives.Int Immunol. 2023 Dec 23;35(12):571-582. doi: 10.1093/intimm/dxad021. Int Immunol. 2023. PMID: 37330692 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
