Dendrimeric Structures in the Synthesis of Fine Chemicals
- PMID: 34576547
- PMCID: PMC8471025
- DOI: 10.3390/ma14185318
Dendrimeric Structures in the Synthesis of Fine Chemicals
Abstract
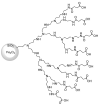
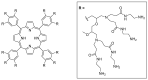
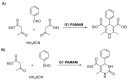
Dendrimers are highly branched structures with a defined shape, dimension, and molecular weight. They consist of three major components: the central core, branches, and terminal groups. In recent years, dendrimers have received great attention in medicinal chemistry, diagnostic field, science of materials, electrochemistry, and catalysis. In addition, they are largely applied for the functionalization of biocompatible semiconductors, in gene transfection processes, as well as in the preparation of nano-devices, including heterogeneous catalysts. Here, we describe recent advances in the design and application of dendrimers in catalytic organic and inorganic processes, sustainable and low environmental impact, photosensitive materials, nano-delivery systems, and antiviral agents' dendrimers.
Keywords: PAMAM; anti-virals; dendrimers; heterogenous catalysis; materials; nano-devices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



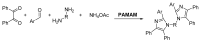



Similar articles
-
Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials.Acc Chem Res. 2012 Apr 17;45(4):630-40. doi: 10.1021/ar200235m. Epub 2011 Dec 8. Acc Chem Res. 2012. PMID: 22148925 Review.
-
Biomedical applications of nano-antioxidant.Methods Mol Biol. 2013;1028:147-51. doi: 10.1007/978-1-62703-475-3_9. Methods Mol Biol. 2013. PMID: 23740118 Review.
-
Nanostructured catalysts for organic transformations.Acc Chem Res. 2013 Aug 20;46(8):1825-37. doi: 10.1021/ar300197s. Epub 2013 Jan 25. Acc Chem Res. 2013. PMID: 23350747 Review.
-
Dendrimeric phosphines in asymmetric catalysis.Chem Soc Rev. 2008 Jan;37(1):56-67. doi: 10.1039/b606569b. Epub 2007 Sep 3. Chem Soc Rev. 2008. PMID: 18197333 Review.
-
Selectively Fluorinated PAMAM-Arginine Conjugates as Gene Delivery Vectors.Bioconjug Chem. 2023 Jun 21;34(6):1084-1095. doi: 10.1021/acs.bioconjchem.3c00139. Epub 2023 May 23. Bioconjug Chem. 2023. PMID: 37221455 Free PMC article.
Cited by
-
mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles.Int J Mol Sci. 2024 Feb 1;25(3):1739. doi: 10.3390/ijms25031739. Int J Mol Sci. 2024. PMID: 38339015 Free PMC article. Review.
-
Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective.Polymers (Basel). 2022 Jun 16;14(12):2462. doi: 10.3390/polym14122462. Polymers (Basel). 2022. PMID: 35746038 Free PMC article. Review.
References
-
- Zimmerman S.C. Dendrimers in molecular recognition and self-assembly. Curr. Opin. Colloid Interface Sci. 1997;2:89–99. doi: 10.1016/S1359-0294(97)80013-1. - DOI
-
- Kummu M., Ward P.J., de Moel H., Varis O. Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ. Res. Lett. 2010;5:34006. doi: 10.1088/1748-9326/5/3/034006. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

