Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-Inflammatory Properties Prepared by Needle-Free Electrospinning
- PMID: 34575602
- PMCID: PMC8464763
- DOI: 10.3390/pharmaceutics13091527
Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-Inflammatory Properties Prepared by Needle-Free Electrospinning
Abstract
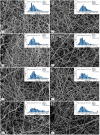
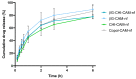
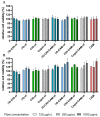
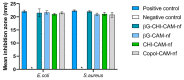
An active wound dressing should address the main goals in wound treatment, which are improved wound healing and reduced infection rates. We developed novel multifunctional nanofibrous wound dressings with three active ingredients: chloramphenicol (CAM), beta-glucan (βG) and chitosan (CHI), of which βG and CHI are active nanofiber-forming biopolymers isolated from the cell walls of Saccharomyces cerevisiae and from shrimp shells, respectively. To evaluate the effect of each active ingredient on the nanofibers' morphological features and bioactivity, nanofibers with both βG and CHI, only βG, only CHI and only copolymers, polyethylene oxide (PEO) and hydroxypropylmethylcellulose (HPMC) were fabricated. All four nanofiber formulations were also prepared with 1% CAM. The needle-free NanospiderTM technique allowed for the successful production of defect-free nanofibers containing all three active ingredients. The CAM-containing nanofibers had a burst CAM-release and a high absorption capacity. Nanofibers with all active ingredients (βG, CHI and CAM) showed a concentration-dependent anti-inflammatory activity, while maintaining the antimicrobial activity of CAM. The promising anti-inflammatory properties, together with the high absorption capacity and antimicrobial effect, make these multifunctional nanofibers promising as dressings in local treatment of infected and exuding wounds, such as burn wounds.
Keywords: NanospiderTM; anti-inflammatory activity; antimicrobial activity; chloramphenicol; electrospinning; nanofiber.
Conflict of interest statement
Rolf Einar Engstad is employed at Biotec BetaGlucans. The company Biotec BetaGlucans had role in the providing Soluble β-1.3/1.6-glucan (SBG®) to this current work. Rolf Einar Engstad has no economical or commercial interest to disclaim. The other authors declare no conflict of interest.
Figures
Similar articles
-
Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice.Eur J Pharm Sci. 2018 Aug 30;121:269-280. doi: 10.1016/j.ejps.2018.05.031. Epub 2018 Jun 1. Eur J Pharm Sci. 2018. PMID: 29864585
-
Antimicrobial liposomes-in-nanofiber wound dressings prepared by a green and sustainable wire-electrospinning set-up.Int J Pharm. 2024 May 25;657:124136. doi: 10.1016/j.ijpharm.2024.124136. Epub 2024 Apr 19. Int J Pharm. 2024. PMID: 38642621
-
Physical and biological properties of blend-electrospun polycaprolactone/chitosan-based wound dressings loaded with N-decyl-N, N-dimethyl-1-decanaminium chloride: An in vitro and in vivo study.J Biomed Mater Res B Appl Biomater. 2020 Nov;108(8):3084-3098. doi: 10.1002/jbm.b.34636. Epub 2020 May 27. J Biomed Mater Res B Appl Biomater. 2020. PMID: 32459395
-
An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings.Mini Rev Med Chem. 2018 Feb 14;18(5):414-427. doi: 10.2174/1389557517666170308112147. Mini Rev Med Chem. 2018. PMID: 28271816 Review.
-
Antibacterial biohybrid nanofibers for wound dressings.Acta Biomater. 2020 Apr 15;107:25-49. doi: 10.1016/j.actbio.2020.02.022. Epub 2020 Feb 19. Acta Biomater. 2020. PMID: 32084600 Review.
Cited by
-
Chitosan-based delivery system enhances antimicrobial activity of chlorhexidine.Front Microbiol. 2022 Sep 29;13:1023083. doi: 10.3389/fmicb.2022.1023083. eCollection 2022. Front Microbiol. 2022. PMID: 36246245 Free PMC article.
-
Imination of Microporous Chitosan Fibers-A Route to Biomaterials with "On Demand" Antimicrobial Activity and Biodegradation for Wound Dressings.Pharmaceutics. 2022 Jan 4;14(1):117. doi: 10.3390/pharmaceutics14010117. Pharmaceutics. 2022. PMID: 35057012 Free PMC article.
-
Fabrication of Nanofibers Based on Hydroxypropyl Starch/Polyurethane Loaded with the Biosynthesized Silver Nanoparticles for the Treatment of Pathogenic Microbes in Wounds.Polymers (Basel). 2022 Jan 13;14(2):318. doi: 10.3390/polym14020318. Polymers (Basel). 2022. PMID: 35054723 Free PMC article.
-
Polysaccharide Electrospun Nanofibers for Wound Healing Applications.Int J Nanomedicine. 2022 Sep 6;17:3913-3931. doi: 10.2147/IJN.S371900. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36097445 Free PMC article. Review.
-
Developing and Screening of a Bacteria-Fighting and Moisture-Controlling Coccinia grandis and Poly(vinyl alcohol) Electrospun Nanofibrous Mat.ACS Omega. 2024 Jul 4;9(28):30926-30934. doi: 10.1021/acsomega.4c03884. eCollection 2024 Jul 16. ACS Omega. 2024. PMID: 39035951 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

