Meiotic Behavior of Achiasmate Sex Chromosomes in the African Pygmy Mouse Mus mattheyi Offers New Insights into the Evolution of Sex Chromosome Pairing and Segregation in Mammals
- PMID: 34573416
- PMCID: PMC8471055
- DOI: 10.3390/genes12091434
Meiotic Behavior of Achiasmate Sex Chromosomes in the African Pygmy Mouse Mus mattheyi Offers New Insights into the Evolution of Sex Chromosome Pairing and Segregation in Mammals
Abstract
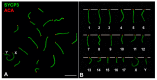
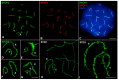
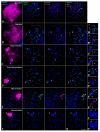
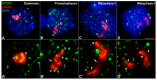
X and Y chromosomes in mammals are different in size and gene content due to an evolutionary process of differentiation and degeneration of the Y chromosome. Nevertheless, these chromosomes usually share a small region of homology, the pseudoautosomal region (PAR), which allows them to perform a partial synapsis and undergo reciprocal recombination during meiosis, which ensures their segregation. However, in some mammalian species the PAR has been lost, which challenges the pairing and segregation of sex chromosomes in meiosis. The African pygmy mouse Mus mattheyi shows completely differentiated sex chromosomes, representing an uncommon evolutionary situation among mouse species. We have performed a detailed analysis of the location of proteins involved in synaptonemal complex assembly (SYCP3), recombination (RPA, RAD51 and MLH1) and sex chromosome inactivation (γH2AX) in this species. We found that neither synapsis nor chiasmata are found between sex chromosomes and their pairing is notably delayed compared to autosomes. Interestingly, the Y chromosome only incorporates RPA and RAD51 in a reduced fraction of spermatocytes, indicating a particular DNA repair dynamic on this chromosome. The analysis of segregation revealed that sex chromosomes are associated until metaphase-I just by a chromatin contact. Unexpectedly, both sex chromosomes remain labelled with γH2AX during first meiotic division. This chromatin contact is probably enough to maintain sex chromosome association up to anaphase-I and, therefore, could be relevant to ensure their reductional segregation. The results presented suggest that the regulation of both DNA repair and epigenetic modifications in the sex chromosomes can have a great impact on the divergence of sex chromosomes and their proper transmission, widening our understanding on the relationship between meiosis and the evolution of sex chromosomes in mammals.
Keywords: Mus mattheyi; evolution; meiosis; pygmy mouse; sex chromosomes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Meiotic pairing and segregation of achiasmate sex chromosomes in eutherian mammals: the role of SYCP3 protein.PLoS Genet. 2007 Nov;3(11):e198. doi: 10.1371/journal.pgen.0030198. PLoS Genet. 2007. PMID: 17983272 Free PMC article.
-
Meiosis reveals the early steps in the evolution of a neo-XY sex chromosome pair in the African pygmy mouse Mus minutoides.PLoS Genet. 2020 Nov 12;16(11):e1008959. doi: 10.1371/journal.pgen.1008959. eCollection 2020 Nov. PLoS Genet. 2020. PMID: 33180767 Free PMC article.
-
Sex chromosome quadrivalents in oocytes of the African pygmy mouse Mus minutoides that harbors non-conventional sex chromosomes.Chromosoma. 2019 Sep;128(3):397-411. doi: 10.1007/s00412-019-00699-4. Epub 2019 Mar 27. Chromosoma. 2019. PMID: 30919035
-
Recent advances in mechanisms ensuring the pairing, synapsis and segregation of XY chromosomes in mice and humans.Cell Mol Life Sci. 2024 Apr 23;81(1):194. doi: 10.1007/s00018-024-05216-0. Cell Mol Life Sci. 2024. PMID: 38653846 Free PMC article. Review.
-
The tricky path to recombining X and Y chromosomes in meiosis.Ann N Y Acad Sci. 2012 Sep;1267:18-23. doi: 10.1111/j.1749-6632.2012.06593.x. Ann N Y Acad Sci. 2012. PMID: 22954211 Free PMC article. Review.
Cited by
-
Strategies for meiotic sex chromosome dynamics and telomeric elongation in Marsupials.PLoS Genet. 2022 Feb 7;18(2):e1010040. doi: 10.1371/journal.pgen.1010040. eCollection 2022 Feb. PLoS Genet. 2022. PMID: 35130272 Free PMC article.
-
Fragile, unfaithful and persistent Ys-on how meiosis can shape sex chromosome evolution.Heredity (Edinb). 2022 Jul;129(1):22-30. doi: 10.1038/s41437-022-00532-2. Epub 2022 Apr 22. Heredity (Edinb). 2022. PMID: 35459933 Free PMC article.
-
X Chromosome Inactivation during Grasshopper Spermatogenesis.Genes (Basel). 2021 Nov 23;12(12):1844. doi: 10.3390/genes12121844. Genes (Basel). 2021. PMID: 34946793 Free PMC article.
-
Synaptonemal & CO analyzer: A tool for synaptonemal complex and crossover analysis in immunofluorescence images.Front Cell Dev Biol. 2023 Jan 19;11:1005145. doi: 10.3389/fcell.2023.1005145. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36743415 Free PMC article.
-
Unusual Mammalian Sex Determination Systems: A Cabinet of Curiosities.Genes (Basel). 2021 Nov 8;12(11):1770. doi: 10.3390/genes12111770. Genes (Basel). 2021. PMID: 34828376 Free PMC article. Review.
References
-
- John B. Meiosis. Cambridge University Press; Cambridge, MA, USA: 1990.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

