Beta vulgaris rubra L. (Beetroot) Peel Methanol Extract Reduces Oxidative Stress and Stimulates Cell Proliferation via Increasing VEGF Expression in H2O2 Induced Oxidative Stressed Human Umbilical Vein Endothelial Cells
- PMID: 34573361
- PMCID: PMC8466581
- DOI: 10.3390/genes12091380
Beta vulgaris rubra L. (Beetroot) Peel Methanol Extract Reduces Oxidative Stress and Stimulates Cell Proliferation via Increasing VEGF Expression in H2O2 Induced Oxidative Stressed Human Umbilical Vein Endothelial Cells
Abstract
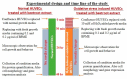
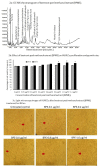
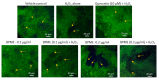
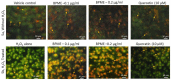
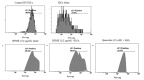
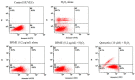
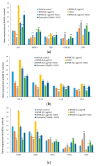
The antioxidant capacity of polyphenols and flavonoids present in dietary agents aids in arresting the development of reactive oxygen species (ROS) and protecting endothelial smooth muscle cells from oxidative stress/induced necrosis. Beetroot (Beta vulgaris var. rubra L.; BVr) is a commonly consumed vegetable representing a rich source of antioxidants. Beetroot peel's bioactive compounds and their role in human umbilical vein endothelial cells (HUVECs) are still under-researched. In the present study, beetroot peel methanol extract (BPME) was prepared, and its effect on the bio-efficacy, nuclear integrity, mitochondrial membrane potential and vascular cell growth, and immunoregulation-related gene expression levels in HUVECs with induced oxidative stress were analysed. Gas chromatography-mass spectroscopy (GC-MS) results confirmed that BPME contains 5-hydroxymethylfurfural (32.6%), methyl pyruvate (15.13%), furfural (9.98%), and 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-Pyran-4-one (12.4%). BPME extract effectively enhanced cell proliferation and was confirmed by MTT assay; the nuclear integrity was confirmed by propidium iodide (PI) staining assay; the mitochondrial membrane potential (Δψm) was confirmed by JC-1 staining assay. Annexin V assay confirmed that BPME-treated HUVECs showed 99% viable cells, but only 39.8% viability was shown in HUVECs treated with H2O2 alone. In addition, BPME treatment of HUVECs for 48 h reduced mRNA expression of lipid peroxide (LPO) and increased NOS-3, Nrf-2, GSK-3β, GPX, endothelial nitric oxide synthase (eNOS) and vascular cell growth factor (VEGF) mRNA expression levels. We found that BPME treatment decreased proinflammatory (nuclear factor-κβ (F-κβ), tissue necrosis factor-α (TNF-α), toll-like receptor-4 (TLR-4), interleukin-1β (IL-1β)) and vascular inflammation (intracellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), EDN1, IL-1β)-related mRNA expressions. In conclusion, beetroot peel treatment effectively increased vascular smooth cell growth factors and microtubule development, whereas it decreased vascular inflammatory regulators. BPME may be beneficial for vascular smooth cell regeneration, tissue repair and anti-ageing potential.
Keywords: angiogenesis; beetroot; inflammation; mitochondria; oxidative stress.
Conflict of interest statement
There is no conflict of interest for this study.
Figures

Similar articles
-
An isoflavonoid-enriched extract from Pueraria lobata (kudzu) root protects human umbilical vein endothelial cells against oxidative stress induced apoptosis.J Ethnopharmacol. 2016 Dec 4;193:524-530. doi: 10.1016/j.jep.2016.10.005. Epub 2016 Oct 4. J Ethnopharmacol. 2016. PMID: 27717903
-
Protective effects of extracts from Ephedra foeminea Forssk fruits against oxidative injury in human endothelial cells.J Ethnopharmacol. 2020 Oct 5;260:112976. doi: 10.1016/j.jep.2020.112976. Epub 2020 May 16. J Ethnopharmacol. 2020. PMID: 32428657
-
Anti-angiogenic effect of Nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potential.PLoS One. 2015 Feb 25;10(2):e0118552. doi: 10.1371/journal.pone.0118552. eCollection 2015. PLoS One. 2015. PMID: 25714482 Free PMC article.
-
Nutritional and functional potential of Beta vulgaris cicla and rubra.Fitoterapia. 2013 Sep;89:188-99. doi: 10.1016/j.fitote.2013.06.004. Epub 2013 Jun 7. Fitoterapia. 2013. PMID: 23751216 Review.
-
Reversibility of endothelial dysfunction in diabetes: role of polyphenols.Br J Nutr. 2016 Jul;116(2):223-46. doi: 10.1017/S0007114516001884. Epub 2016 Jun 6. Br J Nutr. 2016. PMID: 27264638 Review.
References
-
- Abdolmaleki Z., Arab H.-A., Amanpour S., Muhammadnejad S. Anti-angiogenic effects of ethanolic extract of Artemisia sieberi compared to its active substance, artemisinin. Rev. Bras. Farmacogn. 2016;26:326–333. doi: 10.1016/j.bjp.2015.11.008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

