Role of TRP ion channels in cerebral circulation and neurovascular communication
- PMID: 34560190
- PMCID: PMC8572163
- DOI: 10.1016/j.neulet.2021.136258
Role of TRP ion channels in cerebral circulation and neurovascular communication
Abstract
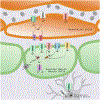
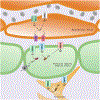
The dynamic regulation of blood flow is essential for meeting the high metabolic demands of the brain and maintaining brain function. Cerebral blood flow is regulated primarily by 1) the intrinsic mechanisms that determine vascular contractility and 2) signals from neurons and astrocytes that alter vascular contractility. Stimuli from neurons and astrocytes can also initiate a signaling cascade in the brain capillary endothelium to increase regional blood flow. Recent studies provide evidence that TRP channels in endothelial cells, smooth muscle cells, neurons, astrocytes, and perivascular nerves control cerebrovascular contractility and cerebral blood flow. TRP channels exert their functional effects either through cell membrane depolarization or by serving as a Ca2+ influx pathway. Endothelial cells and astrocytes also maintain the integrity of the blood-brain barrier. Both endothelial cells and astrocytes express TRP channels, and an increase in endothelial TRP channel activity has been linked with a disrupted endothelial barrier function. Therefore, TRP channels can play a potentially important role in regulating blood-brain barrier integrity. Here, we review the regulation of cerebrovascular contractility by TRP channels under healthy and disease conditions and their potential roles in maintaining blood-brain barrier function.
Keywords: Blood-brain barrier; Cerebral blood flow; Cerebral microcirculation; Neurovascular coupling; TRP channels.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Transient Receptor Potential Channels in the Healthy and Diseased Blood-Brain Barrier.J Histochem Cytochem. 2024 Apr;72(4):199-231. doi: 10.1369/00221554241246032. Epub 2024 Apr 8. J Histochem Cytochem. 2024. PMID: 38590114 Review.
-
Ion channel networks in the control of cerebral blood flow.J Cereb Blood Flow Metab. 2016 Mar;36(3):492-512. doi: 10.1177/0271678X15616138. Epub 2015 Nov 9. J Cereb Blood Flow Metab. 2016. PMID: 26661232 Free PMC article. Review.
-
Transient receptor potential channels in the vasculature.Physiol Rev. 2015 Apr;95(2):645-90. doi: 10.1152/physrev.00026.2014. Physiol Rev. 2015. PMID: 25834234 Free PMC article. Review.
-
[The metabolic regulation of cerebral microcirculation].Rev Neurol. 2007 Apr 1-15;44(7):415-25. Rev Neurol. 2007. PMID: 17420968 Review. Spanish.
-
Targeting endothelial ion signalling to rescue cerebral blood flow in cerebral disorders.Vascul Pharmacol. 2022 Aug;145:106997. doi: 10.1016/j.vph.2022.106997. Epub 2022 May 5. Vascul Pharmacol. 2022. PMID: 35526818
Cited by
-
Impact of aging on vascular ion channels: perspectives and knowledge gaps across major organ systems.Am J Physiol Heart Circ Physiol. 2023 Nov 1;325(5):H1012-H1038. doi: 10.1152/ajpheart.00288.2023. Epub 2023 Aug 25. Am J Physiol Heart Circ Physiol. 2023. PMID: 37624095 Free PMC article. Review.
-
The role of endothelial TRP channels in age-related vascular cognitive impairment and dementia.Front Aging Neurosci. 2023 Mar 20;15:1149820. doi: 10.3389/fnagi.2023.1149820. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37020858 Free PMC article. Review.
-
Micromotion Derived Fluid Shear Stress Mediates Peri-Electrode Gliosis through Mechanosensitive Ion Channels.Adv Sci (Weinh). 2023 Sep;10(27):e2301352. doi: 10.1002/advs.202301352. Epub 2023 Jul 30. Adv Sci (Weinh). 2023. PMID: 37518828 Free PMC article.
-
Lysosomal TRPML1 triggers global Ca2+ signals and nitric oxide release in human cerebrovascular endothelial cells.Front Physiol. 2024 Jun 21;15:1426783. doi: 10.3389/fphys.2024.1426783. eCollection 2024. Front Physiol. 2024. PMID: 38974517 Free PMC article.
-
Transient Receptor Potential Channels in the Healthy and Diseased Blood-Brain Barrier.J Histochem Cytochem. 2024 Apr;72(4):199-231. doi: 10.1369/00221554241246032. Epub 2024 Apr 8. J Histochem Cytochem. 2024. PMID: 38590114 Review.
References
-
- Abdi A, Mazzocco C, Légeron FP, Yvert B, Macrez N, Morel JL, TRPP2 modulates ryanodine- and inositol-1,4,5-trisphosphate receptors-dependent Ca2+ signals in opposite ways in cerebral arteries. Cell Calcium, Vol. 58, 2015, pp. 467–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

