Uteroplacental nutrient flux and evidence for metabolic reprogramming during sustained hypoxemia
- PMID: 34558219
- PMCID: PMC8461030
- DOI: 10.14814/phy2.15033
Uteroplacental nutrient flux and evidence for metabolic reprogramming during sustained hypoxemia
Abstract
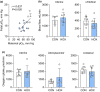
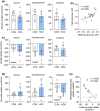
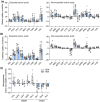
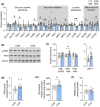
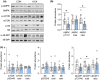
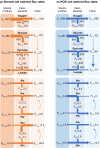
Gestational hypoxemia is often associated with reduced birth weight, yet how hypoxemia controls uteroplacental nutrient metabolism and supply to the fetus is unclear. This study tested the effects of maternal hypoxemia (HOX) between 0.8 and 0.9 gestation on uteroplacental nutrient metabolism and flux to the fetus in pregnant sheep. Despite hypoxemia, uteroplacental and fetal oxygen utilization and net glucose and lactate uptake rates were similar in HOX (n = 11) compared to CON (n = 7) groups. HOX fetuses had increased lactate and pyruvate concentrations and increased net pyruvate output to the utero-placenta. In the HOX group, uteroplacental flux of alanine to the fetus was decreased, as was glutamate flux from the fetus. HOX fetuses had increased alanine and decreased aspartate, serine, and glutamate concentrations. In HOX placental tissue, we identified hypoxic responses that should increase mitochondrial efficiency (decreased SDHB, increased COX4I2) and increase lactate production from pyruvate (increased LDHA protein and LDH activity, decreased LDHB and MPC2), both resembling metabolic reprogramming, but with evidence for decreased (PFK1, PKM2), rather than increased, glycolysis and AMPK phosphorylation. This supports a fetal-uteroplacental shuttle during sustained hypoxemia whereby uteroplacental tissues produce lactate as fuel for the fetus using pyruvate released from the fetus, rather than pyruvate produced from glucose in the placenta, given the absence of increased uteroplacental glucose uptake and glycolytic gene activation. Together, these results provide new mechanisms for how hypoxemia, independent of AMPK activation, regulates uteroplacental metabolism and nutrient allocation to the fetus, which allow the fetus to defend its oxidative metabolism and growth.
Keywords: fetal; hypoxemia; metabolism; uteroplacental.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures
Similar articles
-
Tissue-specific responses that constrain glucose oxidation and increase lactate production with the severity of hypoxemia in fetal sheep.Am J Physiol Endocrinol Metab. 2022 Feb 1;322(2):E181-E196. doi: 10.1152/ajpendo.00382.2021. Epub 2021 Dec 27. Am J Physiol Endocrinol Metab. 2022. PMID: 34957858 Free PMC article.
-
Adaptive responses in uteroplacental metabolism and fetoplacental nutrient shuttling and sensing during placental insufficiency.Am J Physiol Endocrinol Metab. 2023 Jun 1;324(6):E556-E568. doi: 10.1152/ajpendo.00046.2023. Epub 2023 Apr 26. Am J Physiol Endocrinol Metab. 2023. PMID: 37126847 Free PMC article.
-
A physiological increase in maternal cortisol alters uteroplacental metabolism in the pregnant ewe.J Physiol. 2016 Nov 1;594(21):6407-6418. doi: 10.1113/JP272301. Epub 2016 Jul 6. J Physiol. 2016. PMID: 27292274 Free PMC article.
-
New concepts in fetal and placental amino acid metabolism.J Anim Sci. 1992 Oct;70(10):3258-63. doi: 10.2527/1992.70103258x. J Anim Sci. 1992. PMID: 1429302 Review.
-
The physiology of intrapartum fetal compromise at term.Am J Obstet Gynecol. 2020 Jan;222(1):17-26. doi: 10.1016/j.ajog.2019.07.032. Epub 2019 Jul 24. Am J Obstet Gynecol. 2020. PMID: 31351061 Review.
Cited by
-
Tissue-specific responses that constrain glucose oxidation and increase lactate production with the severity of hypoxemia in fetal sheep.Am J Physiol Endocrinol Metab. 2022 Feb 1;322(2):E181-E196. doi: 10.1152/ajpendo.00382.2021. Epub 2021 Dec 27. Am J Physiol Endocrinol Metab. 2022. PMID: 34957858 Free PMC article.
-
Why is human uterine artery blood flow during pregnancy so high?Am J Physiol Regul Integr Comp Physiol. 2022 Nov 1;323(5):R694-R699. doi: 10.1152/ajpregu.00167.2022. Epub 2022 Sep 12. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 36094446 Free PMC article.
-
Increased hepatic glucose production with lower oxidative metabolism in the growth-restricted fetus.JCI Insight. 2024 Apr 30;9(10):e176497. doi: 10.1172/jci.insight.176497. JCI Insight. 2024. PMID: 38687612 Free PMC article.
-
Adaptive responses in uteroplacental metabolism and fetoplacental nutrient shuttling and sensing during placental insufficiency.Am J Physiol Endocrinol Metab. 2023 Jun 1;324(6):E556-E568. doi: 10.1152/ajpendo.00046.2023. Epub 2023 Apr 26. Am J Physiol Endocrinol Metab. 2023. PMID: 37126847 Free PMC article.
-
Absence of Metformin in Fetal Circulation Following Maternal Administration in Late Gestation Pregnant Sheep.Reprod Sci. 2024 Jun;31(6):1763-1766. doi: 10.1007/s43032-024-01547-2. Epub 2024 Apr 23. Reprod Sci. 2024. PMID: 38653860 Free PMC article.
References
-
- Ananth, C. V. (2014). Ischemic placental disease: A unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Seminars in Perinatology, 38, 131–132. - PubMed
-
- Ananth, C. V., & Vintzileos, A. M. (2008). Medically indicated preterm birth: Recognizing the importance of the problem. Clinics in Perinatology, 35, 53–67, viii. - PubMed
-
- Battaglia, F. C. (2000). Glutamine and glutamate exchange between the fetal liver and the placenta. Journal of Nutrition, 130, 974S–977S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

