Deciphering Succession and Assembly Patterns of Microbial Communities in a Two-Stage Solid-State Fermentation System
- PMID: 34549993
- PMCID: PMC8557893
- DOI: 10.1128/Spectrum.00718-21
Deciphering Succession and Assembly Patterns of Microbial Communities in a Two-Stage Solid-State Fermentation System
Abstract
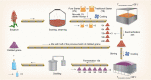
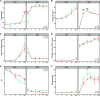
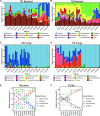
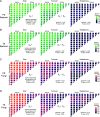
Although the importance of microbiota in the natural environment and in industrial production has been widely recognized, little is known about the formation and succession patterns of the microbial community, particularly secondary succession after disturbance. Here, we choose the Xiaoqu liquor brewing process as an experimental model in which sorghum grains were first aerobically saccharified and then anaerobically fermented after being stirred and acidified to explore multistage community succession patterns. We analyzed microbial composition, physicochemical factors, and metabolites of brewing grains inoculated with two different starters, pure starter and traditional starter, respectively. Two groups showed similar succession patterns where the saccharification microbiota was mainly derived from starters, while environmental microorganisms, mainly Lactobacillaceae and Saccharomyces, dominated the fermentation microbiota regardless of the original saccharification community composition. Species replacement shaped the bacterial community, while species replacement and loss both contributed to fungal community succession in both groups. Grain acidification and hypoxia led to the succession of bacterial and fungal communities during fermentation, respectively. Despite inoculation with starters containing different microorganisms, similar microbial communities during the fermentation stage of the two groups exhibited similar metabolite composition. However, higher abundance of Rhizopus in the saccharification of the pure starter group led to more alcohols, while higher abundance of Monascus and Saccharomycopsis in the traditional starter group promoted acid and ester metabolism. These results revealed the microbial succession patterns of two-stage liquor brewing and its influence on flavor metabolism, which could be used to regulate the microbial community in food fermentation to further promote the modernization of the fermented food industry. IMPORTANCE Revealing formation and assembly mechanisms of microbiota can help us to understand and further regulate its roles in the ecosystems. The Xiaoqu liquor brewing system is a tractable microbial ecosystem with low complexity. This two-stage microbial ecosystem can be used as an experimental model to analyze the multistage temporal succession pattern of microbial communities. Our results demonstrated the dynamic composition and succession pattern of a microbial community in the two-stage liquor brewing system. The results also revealed the microbial origins determining community composition, the ecological processes dominating microbial community succession patterns, the determinants affecting microbial community successions, and the effect of microbial community changes on metabolite synthesis. Overall, our study not only provides an insight into multistage succession patterns of microbial communities in liquor brewing systems but also provides reference for optimizing the quality of fermented products, which will be helpful to understand the succession patterns of microbial communities in other natural ecosystems.
Keywords: Xiaoqu liquor; community assembly; community succession; nestedness; turnover.
Figures

Similar articles
-
Microbial succession and its effect on key aroma components during light-aroma-type Xiaoqu Baijiu brewing process.World J Microbiol Biotechnol. 2022 Jul 21;38(10):166. doi: 10.1007/s11274-022-03353-x. World J Microbiol Biotechnol. 2022. PMID: 35861902
-
Environmental Microbiota Drives Microbial Succession and Metabolic Profiles during Chinese Liquor Fermentation.Appl Environ Microbiol. 2018 Jan 31;84(4):e02369-17. doi: 10.1128/AEM.02369-17. Print 2018 Feb 15. Appl Environ Microbiol. 2018. PMID: 29196296 Free PMC article.
-
Effects of Saccharomycopsis fibuligera and Saccharomyces cerevisiae inoculation on small fermentation starters in Sichuan-style Xiaoqu liquor.Food Res Int. 2020 Nov;137:109425. doi: 10.1016/j.foodres.2020.109425. Epub 2020 Jun 6. Food Res Int. 2020. PMID: 33233107
-
Microbial diversity in jiuqu and its fermentation features: saccharification, alcohol fermentation and flavors generation.Appl Microbiol Biotechnol. 2023 Jan;107(1):25-41. doi: 10.1007/s00253-022-12291-5. Epub 2022 Dec 6. Appl Microbiol Biotechnol. 2023. PMID: 36472652 Review.
-
Deciphering the core microbes and their interactions in spontaneous Baijiu fermentation: A comprehensive review.Food Res Int. 2024 Jul;188:114497. doi: 10.1016/j.foodres.2024.114497. Epub 2024 May 11. Food Res Int. 2024. PMID: 38823877 Review.
Cited by
-
Effects of sorghum varieties on microbial communities and volatile compounds in the fermentation of light-flavor Baijiu.Front Microbiol. 2024 Jul 31;15:1421928. doi: 10.3389/fmicb.2024.1421928. eCollection 2024. Front Microbiol. 2024. PMID: 39144211 Free PMC article.
-
Characteristics and Functions of Dominant Yeasts Together with Their Applications during Strong-Flavor Baijiu Brewing.Foods. 2024 Jul 30;13(15):2409. doi: 10.3390/foods13152409. Foods. 2024. PMID: 39123600 Free PMC article.
-
Comparison of the Correlations of Microbial Community and Volatile Compounds between Pit-Mud and Fermented Grains of Compound-Flavor Baijiu.Foods. 2024 Jan 8;13(2):203. doi: 10.3390/foods13020203. Foods. 2024. PMID: 38254504 Free PMC article.
-
Predicting the final metabolic profile based on the succession-related microbiota during spontaneous fermentation of the starter for Chinese liquor making.mSystems. 2024 Feb 20;9(2):e0058623. doi: 10.1128/msystems.00586-23. Epub 2024 Jan 11. mSystems. 2024. PMID: 38206013 Free PMC article.
-
Insights into the influence of physicochemical parameters on the microbial community and volatile compounds during the ultra-long fermentation of compound-flavor Baijiu.Front Microbiol. 2023 Oct 26;14:1272559. doi: 10.3389/fmicb.2023.1272559. eCollection 2023. Front Microbiol. 2023. PMID: 37965554 Free PMC article.
References
-
- Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vazquez-Baeza Y, Gonzalez A, Morton JT, Mirarab S, Zech Xu Z, Jiang L, Haroon MF, Kanbar J, Zhu Q, Jin Song S, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R, Earth Microbiome Project Consortium. 2017. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551:457–463. doi:10.1038/nature24621. - DOI - PMC - PubMed
-
- Ferrenberg S, O’Neill SP, Knelman JE, Todd B, Duggan S, Bradley D, Robinson T, Schmidt SK, Townsend AR, Williams MW, Cleveland CC, Melbourne BA, Jiang L, Nemergut DR. 2013. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J 7:1102–1111. doi:10.1038/ismej.2013.11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

