Neuroimmune signatures in chronic low back pain subtypes
- PMID: 34528069
- PMCID: PMC9128369
- DOI: 10.1093/brain/awab336
Neuroimmune signatures in chronic low back pain subtypes
Abstract
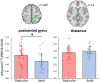
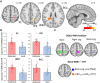
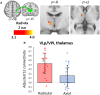
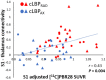
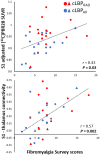
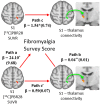
We recently showed that patients with different chronic pain conditions (such as chronic low back pain, fibromyalgia, migraine and Gulf War illness) demonstrated elevated brain and/or spinal cord levels of the glial marker 18-kDa translocator protein (TSPO), which suggests that neuroinflammation might be a pervasive phenomenon observable across multiple aetiologically heterogeneous pain disorders. Interestingly, the spatial distribution of this neuroinflammatory signal appears to exhibit a degree of disease specificity (e.g. with respect to the involvement of the primary somatosensory cortex), suggesting that different pain conditions may exhibit distinct 'neuroinflammatory signatures'. To explore this hypothesis further, we tested whether neuroinflammatory signal can characterize putative aetiological subtypes of chronic low back pain patients based on clinical presentation. Specifically, we explored neuroinflammation in patients whose chronic low back pain either did or did not radiate to the leg (i.e. 'radicular' versus 'axial' back pain). Fifty-four patients with chronic low back pain, 26 with axial back pain [43.7 ± 16.6 years old (mean ± SD)] and 28 with radicular back pain (48.3 ± 13.2 years old), underwent PET/MRI with 11C-PBR28, a second-generation radioligand for TSPO. 11C-PBR28 signal was quantified using standardized uptake values ratio (validated against volume of distribution ratio; n = 23). Functional MRI data were collected simultaneously to the 11C-PBR28 data (i) to functionally localize the primary somatosensory cortex back and leg subregions; and (ii) to perform functional connectivity analyses (in order to investigate possible neurophysiological correlations of the neuroinflammatory signal). PET and functional MRI measures were compared across groups, cross-correlated with one another and with the severity of 'fibromyalgianess' (i.e. the degree of pain centralization, or 'nociplastic pain'). Furthermore, statistical mediation models were used to explore possible causal relationships between these three variables. For the primary somatosensory cortex representation of back/leg, 11C-PBR28 PET signal and functional connectivity to the thalamus were: (i) higher in radicular compared to axial back pain patients; (ii) positively correlated with each other; (iii) positively correlated with fibromyalgianess scores, across groups; and finally (iv) fibromyalgianess mediated the association between 11C-PBR28 PET signal and primary somatosensory cortex-thalamus connectivity across groups. Our findings support the existence of 'neuroinflammatory signatures' that are accompanied by neurophysiological changes and correlate with clinical presentation (in particular, with the degree of nociplastic pain) in chronic pain patients. These signatures may contribute to the subtyping of distinct pain syndromes and also provide information about interindividual variability in neuroimmune brain signals, within diagnostic groups, that could eventually serve as targets for mechanism-based precision medicine approaches.
Keywords: chronic pain; functional connectivity; glial cells; inflammation; neuropathic.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Brain glial activation in fibromyalgia - A multi-site positron emission tomography investigation.Brain Behav Immun. 2019 Jan;75:72-83. doi: 10.1016/j.bbi.2018.09.018. Epub 2018 Sep 14. Brain Behav Immun. 2019. PMID: 30223011 Free PMC article.
-
Evidence for brain glial activation in chronic pain patients.Brain. 2015 Mar;138(Pt 3):604-15. doi: 10.1093/brain/awu377. Epub 2015 Jan 12. Brain. 2015. PMID: 25582579 Free PMC article.
-
The neuroinflammatory component of negative affect in patients with chronic pain.Mol Psychiatry. 2021 Mar;26(3):864-874. doi: 10.1038/s41380-019-0433-1. Epub 2019 May 28. Mol Psychiatry. 2021. PMID: 31138890 Free PMC article.
-
Neuroinflammation PET imaging of the translocator protein (TSPO) in Alzheimer's disease: An update.Eur J Neurosci. 2022 Mar;55(5):1322-1343. doi: 10.1111/ejn.15613. Epub 2022 Feb 10. Eur J Neurosci. 2022. PMID: 35083791 Review.
-
Structural, Functional and Neurochemical Cortical Brain Changes Associated with Chronic Low Back Pain.Tomography. 2022 Aug 25;8(5):2153-2163. doi: 10.3390/tomography8050180. Tomography. 2022. PMID: 36136876 Free PMC article. Review.
Cited by
-
The Emotion Regulation of Acupuncture in Chronic Low Back Pain: A Clinical Neuroimaging Protocol.J Pain Res. 2024 Mar 1;17:817-825. doi: 10.2147/JPR.S450589. eCollection 2024. J Pain Res. 2024. PMID: 38444878 Free PMC article.
-
Energy metabolism disturbance in migraine: From a mitochondrial point of view.Front Physiol. 2023 Apr 13;14:1133528. doi: 10.3389/fphys.2023.1133528. eCollection 2023. Front Physiol. 2023. PMID: 37123270 Free PMC article. Review.
-
Association between nociplastic pain and premature endocrine therapy discontinuation in breast cancer patients.Breast Cancer Res Treat. 2023 Jan;197(2):397-404. doi: 10.1007/s10549-022-06806-x. Epub 2022 Nov 13. Breast Cancer Res Treat. 2023. PMID: 36371776 Free PMC article.
-
Unraveling the interplay of neuroinflammatory signaling between parenchymal and meningeal cells in migraine headache.J Headache Pain. 2024 Jul 31;25(1):124. doi: 10.1186/s10194-024-01827-x. J Headache Pain. 2024. PMID: 39080518 Free PMC article. Review.
-
Systemic low-grade C-reactive protein is associated with proximal symptom spread in carpal tunnel syndrome.Pain Rep. 2024 Apr 10;9(3):e1156. doi: 10.1097/PR9.0000000000001156. eCollection 2024 Jun. Pain Rep. 2024. PMID: 38606315 Free PMC article.
References
-
- Mannelli LDC, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain. 2013;14(12):1585–1600. - PubMed
-
- Miyamoto K, Kume K, Ohsawa M. Role of microglia in mechanical allodynia in the anterior cingulate cortex. J Pharmacol Sci. 2017;134(3):158–165. - PubMed
-
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. . P2X 4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. - PubMed
-
- Watkins LR, Hutchinson MR, Johnson KW. Commentary on Landry et al.: “Propentofylline, a CNS glial modulator, does not decrease pain in post-herpetic neuralgia patients: In vitro evidence for differential responses in human and rodent microglia and macrophages”. Exp Neurol. 2012;234(2):351–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

