Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study
- PMID: 34514454
- PMCID: PMC8418937
- DOI: 10.1016/j.lanepe.2021.100208
Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study
Abstract
Background: SARS-CoV-2 mRNA vaccines have proven high efficacy, however, limited data exists on the duration of immune responses and their relation to age and side effects.
Methods: We studied the antibody and memory T cell responses after the two-dose BNT162b2 vaccine in 122 volunteers up to 6 months and correlated the findings with age and side effects.
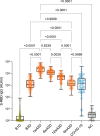
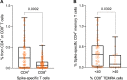
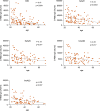
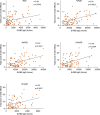
Findings: We found a robust antibody response to Spike protein after the second dose. However, the antibody levels declined at 12 weeks and 6 months post-vaccination, indicating a waning of the immune response over time. At 6 months after the second dose, the Spike antibody levels were similar to the levels in persons vaccinated with one dose or in COVID-19 convalescent individuals. The antibodies efficiently blocked ACE2 receptor binding to SARS-CoV-2 Spike protein of five variants of concern at one week but this was decreased at three months. 87% of individuals developed Spike-specific memory T cell responses, which were lower in individuals with increased proportions of immunosenescent CD8+ TEMRA cells. We found antibody response to correlate negatively with age and positively with the total score of vaccination side effects.
Interpretation: The mRNA vaccine induces a strong antibody response to SARS-CoV-2 and five VOCs at 1 week post-vaccination that decreases thereafter. T cell responses, although detectable in the majority, were lower in individuals with higher T cell immunosenescence. The deterioration of vaccine response suggests the need to monitor for the potential booster vaccination.
Keywords: SARS-CoV-2 mRNA vaccine; adverse effects; age; dynamics of the immune response.
© 2021 The Authors.
Conflict of interest statement
The authors have nothing to disclose.
Figures

Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Waning of specific antibodies against Delta and Omicron variants five months after a third dose of BNT162b2 SARS-CoV-2 vaccine in elderly individuals.Front Immunol. 2022 Nov 14;13:1031852. doi: 10.3389/fimmu.2022.1031852. eCollection 2022. Front Immunol. 2022. PMID: 36451833 Free PMC article.
-
Long-term quantitative assessment of anti-SARS-CoV-2 spike protein immunogenicity (QUASI) after COVID-19 vaccination in older people living with HIV (PWH).BMC Infect Dis. 2022 Sep 21;22(1):744. doi: 10.1186/s12879-022-07737-0. BMC Infect Dis. 2022. PMID: 36131232 Free PMC article.
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
Vaccine-Induced Antibody Responses against SARS-CoV-2 Variants-Of-Concern Six Months after the BNT162b2 COVID-19 mRNA Vaccination.Microbiol Spectr. 2022 Apr 27;10(2):e0225221. doi: 10.1128/spectrum.02252-21. Epub 2022 Mar 9. Microbiol Spectr. 2022. PMID: 35262410 Free PMC article.
Cited by
-
Reduced Antibody Acquisition with Increasing Age following Vaccination with BNT162b2: Results from Two Longitudinal Cohort Studies in The Netherlands.Vaccines (Basel). 2022 Sep 6;10(9):1480. doi: 10.3390/vaccines10091480. Vaccines (Basel). 2022. PMID: 36146557 Free PMC article.
-
Inactivated rabies-vectored SARS-CoV-2 vaccine provides long-term immune response unaffected by vector immunity.NPJ Vaccines. 2022 Sep 23;7(1):110. doi: 10.1038/s41541-022-00532-7. NPJ Vaccines. 2022. PMID: 36151100 Free PMC article.
-
Associations of neuralgic amyotrophy with COVID-19 vaccination: Disproportionality analysis using the World Health Organization pharmacovigilance database.Muscle Nerve. 2022 Dec;66(6):766-770. doi: 10.1002/mus.27734. Epub 2022 Oct 26. Muscle Nerve. 2022. PMID: 36214181 Free PMC article.
-
Systems biology analysis reveals distinct molecular signatures associated with immune responsiveness to the BNT162b COVID-19 vaccine.EBioMedicine. 2024 Jan;99:104947. doi: 10.1016/j.ebiom.2023.104947. Epub 2023 Dec 30. EBioMedicine. 2024. PMID: 38160529 Free PMC article.
-
Reactogenicity and immunogenicity of the intradermal administration of BNT162b2 mRNA vaccine in healthy adults who were primed with an inactivated SARS-CoV-2 vaccine.Vaccine X. 2022 Nov 17;12:100242. doi: 10.1016/j.jvacx.2022.100242. eCollection 2022 Dec. Vaccine X. 2022. PMID: 36415450 Free PMC article.
References
-
- Shrotri M, Fragaszy E, Geismar C, et al. Spike-antibody responses following first and second doses of ChAdOx1 and BNT162b2 vaccines by age, gender, and clinical factors - a prospective community cohort study (Virus Watch) medRxiv. 2021 2021.05.12.21257102.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

