Automated microscope-independent fluorescence-guided micropipette
- PMID: 34513218
- PMCID: PMC8407805
- DOI: 10.1364/BOE.431372
Automated microscope-independent fluorescence-guided micropipette
Abstract
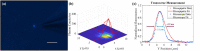
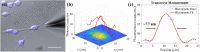
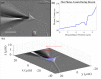
Glass micropipette electrodes are commonly used to provide high resolution recordings of neurons. Although it is the gold standard for single cell recordings, it is highly dependent on the skill of the electrophysiologist. Here, we demonstrate a method of guiding micropipette electrodes to neurons by collecting fluorescence at the aperture, using an intra-electrode tapered optical fiber. The use of a tapered fiber for excitation and collection of fluorescence at the micropipette tip couples the feedback mechanism directly to the distance between the target and electrode. In this study, intra-electrode tapered optical fibers provide a targeted robotic approach to labeled neurons that is independent of microscopy.
© 2021 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Optical method for automated measurement of glass micropipette tip geometry.Precis Eng. 2016 Oct;46:88-95. doi: 10.1016/j.precisioneng.2016.04.003. Epub 2016 Apr 2. Precis Eng. 2016. PMID: 27672230 Free PMC article.
-
Automated navigation of a glass micropipette on a high-density microelectrode array.Annu Int Conf IEEE Eng Med Biol Soc. 2015 Aug;2015:881-4. doi: 10.1109/EMBC.2015.7318503. Annu Int Conf IEEE Eng Med Biol Soc. 2015. PMID: 26736403
-
Efficient nitrogen-vacancy centers' fluorescence excitation and collection from micrometer-sized diamond by a tapered optical fiber in endoscope-type configuration.Opt Express. 2019 Mar 4;27(5):6734-6745. doi: 10.1364/OE.27.006734. Opt Express. 2019. PMID: 30876253
-
Micro-Surface and -Interfacial Tensions Measured Using the Micropipette Technique: Applications in Ultrasound-Microbubbles, Oil-Recovery, Lung-Surfactants, Nanoprecipitation, and Microfluidics.Micromachines (Basel). 2019 Feb 1;10(2):105. doi: 10.3390/mi10020105. Micromachines (Basel). 2019. PMID: 30717224 Free PMC article. Review.
-
In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. PMID: 26844322 Free Books & Documents. Review.
References
-
- Vélez-Fort M., Rousseau C. V., Niedworok C. J., Wickersham I. R., Rancz E. A., Brown A. P., Strom M., Margrie T. W., “The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing,” Neuron 83(6), 1431–1443 (2014).10.1016/j.neuron.2014.08.001 - DOI - PMC - PubMed
-
- Holst G. L., Stoy W., Yang B., Kolb I., Kodandaramaiah S. B., Li L., Knoblich U., Zeng H., Haider B., Boyden E. S., Forest C. R., “Autonomous patch-clamp robot for functional characterization of neurons in vivo: development and application to mouse visual cortex,” J. Neurophysiol. 121(6), 2341–2357 (2019).10.1152/jn.00738.2018 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
