The effect of turn residues on the folding and cell-penetrating activity of β-hairpin peptides and applications toward protein delivery
- PMID: 34504991
- PMCID: PMC8425381
- DOI: 10.1002/pep2.24125
The effect of turn residues on the folding and cell-penetrating activity of β-hairpin peptides and applications toward protein delivery
Abstract
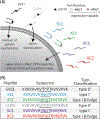
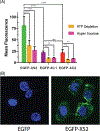
Cell-penetrating peptides (CPPs) are useful tools for the delivery of a wide variety of cargo into cells. Our lab has developed two classes of CPPs based on β-hairpin sequences, one that folds at the surface of cell membranes and the other that is intrinsically disordered. Although these peptides can effectively deliver different types of cargo, their use in protein delivery has been hindered due to the presence of non-natural D-proline within the central turn region of both sequences, which prohibits functionalizing proteins with the CPPs via standard expression protocols. In this work, we describe new CPPs that replace the non-natural turn region with natural turn motifs amenable to protein expression. We first investigate how these changes within the turn affect various CPP-related properties in the absence of protein cargo, and then generate protein fusions for intracellular delivery.
Keywords: cell-penetrating peptides; protein delivery; β-hairpin.
Figures
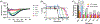
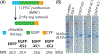
Similar articles
-
Recent Advances of Cell-Penetrating Peptides and Their Application as Vectors for Delivery of Peptide and Protein-Based Cargo Molecules.Pharmaceutics. 2023 Aug 7;15(8):2093. doi: 10.3390/pharmaceutics15082093. Pharmaceutics. 2023. PMID: 37631307 Free PMC article. Review.
-
Peptide internalization enabled by folding: triple helical cell-penetrating peptides.J Pept Sci. 2015 Feb;21(2):77-84. doi: 10.1002/psc.2725. Epub 2014 Dec 18. J Pept Sci. 2015. PMID: 25524829 Free PMC article.
-
Cell penetrating peptides: the potent multi-cargo intracellular carriers.Expert Opin Drug Deliv. 2019 Nov;16(11):1227-1258. doi: 10.1080/17425247.2019.1676720. Epub 2019 Oct 15. Expert Opin Drug Deliv. 2019. PMID: 31583914 Review.
-
Delivery of Various Cargos into Cancer Cells and Tissues via Cell-Penetrating Peptides: A Review of the Last Decade.Pharmaceutics. 2021 Sep 2;13(9):1391. doi: 10.3390/pharmaceutics13091391. Pharmaceutics. 2021. PMID: 34575464 Free PMC article. Review.
-
Expression of Cell-Penetrating Peptides Fused to Protein Cargo.J Mol Microbiol Biotechnol. 2018;28(4):159-168. doi: 10.1159/000494084. Epub 2018 Dec 19. J Mol Microbiol Biotechnol. 2018. PMID: 30566948
Cited by
-
Uncoupling the Folding-Function Paradigm of Lytic Peptides to Deliver Impermeable Inhibitors of Intracellular Protein-Protein Interactions.J Am Chem Soc. 2020 Nov 25;142(47):19950-19955. doi: 10.1021/jacs.0c07921. Epub 2020 Nov 11. J Am Chem Soc. 2020. PMID: 33175531 Free PMC article.
-
Stapled β-Hairpins Featuring 4-Mercaptoproline.J Am Chem Soc. 2021 Sep 22;143(37):15039-15044. doi: 10.1021/jacs.1c04378. Epub 2021 Sep 13. J Am Chem Soc. 2021. PMID: 34516087 Free PMC article.
-
Selection of Nucleotide-Encoded Mass Libraries of Macrocyclic Peptides for Inaccessible Drug Targets.Chem Rev. 2024 Nov 13;124(21):12213-12241. doi: 10.1021/acs.chemrev.4c00422. Epub 2024 Oct 25. Chem Rev. 2024. PMID: 39451037 Free PMC article. Review.
-
Passive Membrane Permeability of Sizable Acyclic β-Hairpin Peptides.ACS Med Chem Lett. 2023 Jan 27;14(3):278-284. doi: 10.1021/acsmedchemlett.2c00486. eCollection 2023 Mar 9. ACS Med Chem Lett. 2023. PMID: 36923919 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
