Sema4C modulates the migration of primary tumor-associated lymphatic endothelial cells via an ERK-mediated pathway
- PMID: 34504556
- PMCID: PMC8383750
- DOI: 10.3892/etm.2021.10535
Sema4C modulates the migration of primary tumor-associated lymphatic endothelial cells via an ERK-mediated pathway
Abstract
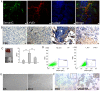
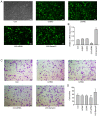
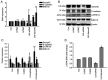
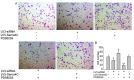
Although lymphatic endothelial cells (LECs) serve a positive role in tumor lymphatic metastasis, the regulation of LECs undergoing migration similar to that of tumor cells remains poorly understood. A previous study revealed that semaphorin 4C (Sema4C) could be a marker of LECs in cervical cancer. Thus, the present study aimed to understand the mechanism via which Sema4C could promote the development of tumor-associated characteristics in LECs in cervical cancer. Primary tumor-associated LECs (TLECs) were distinguished from cervical cancer by flow cytometry. The promigratory ability was assessed using the Transwell assay. Lentivirus infection was used to alter the expression of Sema4C in TLECs. Confocal laser scanning was used to determine the infection efficiency of lentivirus infection. Sema4C/ERK/E-cadherin pathway was measured by reverse transcription-quantitative PCR and western blotting. The co-localization of Sema4C and the lymphatic marker lymphatic vessel endothelial hyaluronan receptor 1 was verified. Primary tumor-associated LECs (TLECs) were isolated from a mouse xenograft cervical tumor model. It was revealed that overexpressing Sema4C stimulated the migratory ability of TLECs, downregulated E-cadherin expression and stimulated ERK phosphorylation, whereas knocking down Sema4C had the opposite effects. The treatment of PD98059 (ERK inhibitor) blocked the pro-migratory ability of TLECs, which indicated a dependence on the ERK signaling pathway. It was identified that the Sema4C/ERK/E-cadherin pathway may be critical for the migration of TLECs, which may promote lymph node metastasis. Therefore, Sema4C could be a promising target for the treatment of cervical cancer with lymphatic metastasis.
Keywords: E-cadherin; extracellular signal-regulated kinase; semaphorin 4C; tumor-associated lymphatic endothelial cells.
Copyright: © Peng et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Tumor-associated Lymphatic Endothelial Cells Promote Lymphatic Metastasis By Highly Expressing and Secreting SEMA4C.Clin Cancer Res. 2017 Jan 1;23(1):214-224. doi: 10.1158/1078-0432.CCR-16-0741. Epub 2016 Jul 8. Clin Cancer Res. 2017. PMID: 27401250
-
Downregulation of SEMA4C Inhibit Epithelial-Mesenchymal Transition (EMT) and the Invasion and Metastasis of Cervical Cancer Cells via Inhibiting Transforming Growth Factor-beta 1 (TGF-β1)-Induced Hela cells p38 Mitogen-Activated Protein Kinase (MAPK) Activation.Med Sci Monit. 2020 Jan 17;26:e918123. doi: 10.12659/MSM.918123. Med Sci Monit. 2020. PMID: 31951596 Free PMC article.
-
Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells.J Exp Clin Cancer Res. 2023 Jul 6;42(1):160. doi: 10.1186/s13046-023-02714-0. J Exp Clin Cancer Res. 2023. PMID: 37415190 Free PMC article.
-
[Effects of vascular endothelial growth factor D on the signaling cascade of sentinel lymphatic endothelial cells from melanoma patients undergoing sentinel lymphadenectomy].Gan To Kagaku Ryoho. 2003 Dec;30(13):2141-4. Gan To Kagaku Ryoho. 2003. Retraction in: Gan To Kagaku Ryoho. 2004 Apr;31(4):678. PMID: 14712780 Retracted. Review. Japanese.
-
A short review on lymphatic endothelial cell heterogeneity.Inflamm Regen. 2021 Oct 11;41(1):32. doi: 10.1186/s41232-021-00183-6. Inflamm Regen. 2021. PMID: 34635187 Free PMC article. Review.
Cited by
-
Cervical Cancer Imaging Features Associated With ADRB1 as a Risk Factor for Cerebral Neurovascular Metastases.Front Neurol. 2022 Jul 12;13:905761. doi: 10.3389/fneur.2022.905761. eCollection 2022. Front Neurol. 2022. PMID: 35903112 Free PMC article.
-
CD45- erythroid progenitor cells promote lymph node metastasis in gastric cancer by inducing a hybrid epithelial/mesenchymal state in lymphatic endothelial cells.Gastric Cancer. 2023 Nov;26(6):918-933. doi: 10.1007/s10120-023-01425-x. Epub 2023 Sep 7. Gastric Cancer. 2023. PMID: 37676622
-
Tumor microenvironment promotes lymphatic metastasis of cervical cancer: its mechanisms and clinical implications.Front Oncol. 2023 May 10;13:1114042. doi: 10.3389/fonc.2023.1114042. eCollection 2023. Front Oncol. 2023. PMID: 37234990 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
